Influenza is an acute viral respiratory disease in its sporadic, epidemic, or pandemic form, resulting in significant morbidity and mortality in humans [, ]. In the past 107 years, influenza A virus (IAV) has caused 4 pandemics—in 1918 (H1N1), 1957 (H2N2), 1968 (H3N2), and 2009 (H1N1pdm09)—and many epidemics []. Such long-term epidemiologic success of IAVs is due to the high error rate of the RNA polymerase, the tolerance of its surface proteins to mutation, and reassortment of viral RNA segments during coinfections, which can result in antigenic drifts causing epidemics or shifts causing pandemics [, ].
Hemagglutinin (HA) is the major surface glycoprotein of IAV and contains the receptor binding site (RBS) on the globular head domain, which is required for viral entry into host epithelial cells []. Influenza vaccination largely induces neutralizing antibody (Ab) targeting the epitopes in and around the RBS, which can be detected by hemagglutination inhibition (HAI) assay []. The HAI-Ab is a major correlate of protection against influenza []. IAVs rapidly mutate to evade neutralizing Abs, which necessitates constant reformulation of vaccines; thus, monitoring genetic and antigenic changes in the HA is essential for annual vaccine updates [].
A(H1N1) IAV caused the most catastrophic pandemic in 1918, continued to circulate from 1918 to 1957, and disappeared in 1957 [, ]. In 1977, A/USSR/90/1977 (US/77)–like A(H1N1) IAVs reemerged and infected people born after 1952 from 1977 to 1985 [, ]. In 1986, A/Taiwan/1/1986 (TW/86)–like A(H1N1) IAVs, which had dramatic antigenic and genetic differences from US/77-like IAVs, replaced the US/77-like IAVs [, ]. Interestingly, TW/86-like IAVs also caused disease mainly in individuals born after 1952, indicating that many US/77-primed adults were further infected with TW/86-like IAVs. In 1995, antigenically drifted A/Beijing/262/95 (BJ/95)–like viruses, containing a characteristic deletion at HA 130 (H1 numbering), were isolated in Asia []. BJ/95-like IAVs did not circulate in the Americas until they had evolved to A/New Caledonia/20/1999 (NC/99)–like viruses with additional mutations in and around the RBS []. Most of the US population born between 1996 and 2008 was primed with 1999–2008 A(H1N1) IAVs possessing the HA-130 deletion [, ]. In 2009, 1977–2008 A(H1N1) IAVs were replaced by A/California/07/2009 (CA/09) A(H1N1)pdm09 IAV [, ].
First exposure with US/77-like viruses from 1977 to 1985 or TW/86-like viruses from 1986 to 1998 has a profound effect on age- and epitope-specific HAI-Ab responses to CA/09-like viruses [], a phenomenon called “original antigenic sin” []. First infection or vaccination with CA/09-like viruses in 2009 recalled memory B cells (MBCs) to produce lateral patch-targeted HAI-Abs involving HA K163 (K163-Abs) with or without the RBS-targeted HAI-Abs involving K130 (K130-Abs) in some adults primed with US/77-like virus [, , ]. By contrast, first infection with CA/09-like viruses recalled MBCs to produce HAI-Abs targeting the epitope possessing D127 and K130 in adults primed with TW/86-like virus [, , , ].
Exposures with CA/09-like A(H1N1)pdm09 IAVs can result in HAI-Ab immunodominance (HAI-Ab-ID), previously referred as “focused” HAI-Ab responses or “skewed” HAI-Ab responses in some adults [, , ]. Such HAI-Ab-ID may drive virus and Ab evolution by Ab-mediated immune selection and suppression, respectively []. Unfortunately, due to the relatively small numbers of study participants and limited epitopes addressed, these studies were not powered to comprehensively assess levels of CA/09-induced epitope-specific HAI-Ab-ID. Here, we explore the age- and epitope-specific HAI-Ab-ID in 300 adults before and after immunization with CA/09-like vaccine between 2010 and 2016. We also summarized HA evolution of A(H1N1)pdm09 viruses circulated from 2010 to August 2024. Our data indicate that age- and epitope-specific HAI-Ab-IDs are common phenomenon in adults and may have directed A(H1N1)pdm09 virus and HAI-Ab evolution. To possibly predict antigenic drift with the goal of improving vaccine strain selection and guiding vaccine use, it is essential to identify HAI-Ab-IDs and map their binding epitopes at the population level.
METHODS
Serum Samples
Adults were immunized with commercial egg-derived inactivated trivalent influenza vaccine containing A/California/07/2009-PR8 virus from 2010 to 2016. Serum samples were collected at prevaccination and 21 to 28 days postvaccination from 6 seasons. These anonymized samples were available from residual specimens acquired through an organization contracted with the US Centers for Disease Control and Prevention, and their use was approved by human subject research determination review.
Influenza Viruses
IAVs were propagated in embryonated eggs or Madin-Darby canine kidney cells. Some viruses were purified on a linear sucrose gradient []. The following were used: X179A vaccine strain containing CA/09 HA with Q223R egg-adapted mutation, 2 egg- and cell-propagated 2015 A(H1N1)pdm09 viruses, 10 historic A(H1N1) viruses representing all major antigenic clusters that circulated from 1977 to 2008, and a reverse genetics (RG) virus containing 1918 A(H1N1) virus HA []. Four RG viruses were used that contained HA and neuraminidase genes from CA/09 and 6 internal genes from A/Puerto Rico/8/1934, as generated for previous studies [, ]; they possessed either wild type HA (RG-wt) or HA with a single K163Q mutation (RG-K163Q), K130 deletion (RG-130D), or double D127N-N129T mutations to add a glycosylation motif at 127 to 129 (RG-127G). All IAVs are described in Table 1.
HAI Assay
Sera were treated with receptor-destroying enzyme (Denke-Seiken) to remove nonspecific inhibitors and adsorbed with packed turkey red blood cells to remove nonspecific agglutinins prior to testing with 4 HA units of virus and 0.5% turkey red blood cells [].
Ab Adsorption Assay
Serum was mixed with ∼105 HA units of purified virus or PBS as a control. After incubation for 2 hours at 4 °C, the virus-serum mixture was centrifuged for 45 minutes at 100 000g to remove virus-Ab complexes and most unbound viruses. Residual viruses were removed by the addition of 100 µL of packed turkey red blood cells [].
Sequence and Structural Analysis
Genetic sequences of A(H1N1)pdm09 HA that were available in the EpiFlu database of GISAID (Global Initiative on Sharing All Influenza Data) and collected from 2010 to 2024 were aligned. Observed amino acid frequencies were calculated for residues 127, 129, 130, and 163 (H1 numbering). The HA monomer was modeled with PyMOL version 2.1.1 from A/California/04/2009 (PDB:5K9O). Positions of observed amino acid mutations were color coded.
Statistical Analyses
Paired t tests were used to compare geometric mean titers (GMTs). P < .05 was considered statistically significant.
RESULTS
Epitope-Specific HAI-Ab-IDs
Our previous study demonstrated that during the 2009 pandemic, >70% of adults with CA/09-like virus infection who were critically ill displayed HAI-Ab-IDs that targeted different epitopes involving K163, D127, N129, and/or K130 in or near the RBS []. Such HAI-Ab-IDs were determined by using RG-wt control and 3 RG mutants containing a K163Q mutation (RG-K163Q), D127N + N129T double mutations (RG-127G), or a K130 deletion (RG-130D). Here, using the same RG viruses, we determined HAI-Ab-IDs in adults (birth year range [BYR], 1961–1998; age range, 18–49 years) before and 21 to 28 days after immunization with egg-grown trivalent influenza vaccine containing CA/09-like virus HA between 2010 and 2016 (Table 1, Supplementary Table 1).
Sera from 300 donors were first analyzed in HAI assays by using 2 viruses: X179A vaccine strain and RG-wt. HAI-Ab-ID targeting an egg-adapted epitope (R223-Ab-ID) was defined by ≥4-fold reductions in HAI-Ab titers against RG-wt (Q223) as compared with X179A (Q223R). Donors without R223-Ab-ID (n = 260) were investigated in HAI assays with RG-wt, RG-K163Q, RG-127G, and RG-130D. Epitope-specific HAI-Ab-IDs were defined by ≥4-fold reductions of HAI-Ab titers against RG mutants as compared with RG-wt, which indicated that ≥75% HAI-Abs targeted a single epitope. Four types of HAI-Ab-IDs—which targeted the epitopes possessing K163 (K163-Ab-ID), D127 + N129 + K130 (D127/N129/K130-Ab-ID), K130 (K130-Ab-ID), or D127 + N129 (D127/N129-Ab-IDs)—were observed in 27% (71/260) of prevaccination sera (pre-sera) and 40% (105/260) of postvaccination sera (post-sera) of >50% (131/260) of donors (Figure 1, Supplementary Table 1). These data indicated that HAI-Ab-IDs are common phenomena in adults before and after immunization with influenza vaccines containing CA/09 as the H1 component between 2010 and 2016.
Figure 1
Epitope- and age-specific HAI-Ab-IDs. Prevaccination sera (pre-sera) and postvaccination sera (post-sera) were collected from adults (n = 260) immunized with CA/09-like vaccine during 2010 to 2016. The sera were tested in HAI assays with RG-wt, RG-K163Q, RG-130D, and RG-127G viruses. A, Proportions and birth year distribution of 4 types of epitope- and age-specific HAI-Ab-IDs in pre- and post-sera. K163-Ab-IDs were present in middle-aged adults born between 1961 and 1983 [, , ]. D127/N129/K130-Ab-IDs were present in adults born between 1967 and 1993 []. D127/N129-Ab-ID was observed in young adults born between 1980 and 1998. While K130-Ab-IDs were seen in various ages of adults. B, CA/09 HA1 monomer 3-dimensional structure. Abbreviations: HAI-Ab-ID, hemagglutination inhibition antibody immunodominance; RG, reverse genetics; wt, wild type.
Evolution of A(H1N1)pdm09 Viruses and HAI-Abs
To determine whether these HAI-Ab-IDs exert immune selection pressures on the viruses, we compared 100 277 available A(H1N1)pdm09 HA sequences collected from 2010 to 2024 with the 2009 CA/09 HA sequence (Figure 2A). We found that infrequent mutations occurred at the 4 HA positions of 127 (D to E/N/G/A), 129 (N to D/E/K/G/S/Y/T/I/F), 130 (K to N/R/S/H/E/Q/I/T/D), and 163 (K to Q/E/N/R/T/I/L/H) initially after the 2009 pandemic. Over time, 3 antigenic variants became fixed in the 2013–2014 season (K163Q; transition time [TT], 4 years), 2020–2021 season (N129D; TT, 11 years), and 2022–2023 season (K130N; TT, 13 years). Our data indicated that HAI-Ab-IDs to these epitopes were present in the population and likely drove A(H1N1)pdm09 HA evolution by epitope-specific HAI-Ab–mediated immune selection.
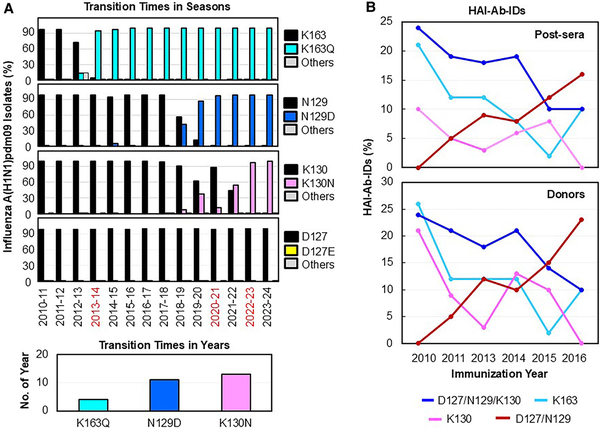
Figure 2
Evolutions of A(H1N1)pdm09 field strains and HAI-Ab-IDs. A, Transition times in seasons were defined by the mutations of K163Q, N129D, and K130N first reaching their near fixation (>90%) within a season in a viral population. The HA sequences (n = 100 277) were obtained from routine influenza surveillance from the following seasons: 2010–2011 (n = 3692), 2011–2012 (n = 1200), 2012–2013 (n = 2556), 2013–2014 (n = 3112), 2014–2015 (n = 1823), 2015–2016 (n = 8653), 2016–2017 (n = 2071), 2017–2018 (n = 7066), 2018–2019 (n = 13 335), 2019–2020 (n = 9682), 2020–2021 (n = 298), 2021–2022 (n = 2388), 2022–2023 (n = 18 085), and 2023–2024 (n = 26 316). Percentage of field strains that possessed amino acid substitutions at the 4 HA positions from the 2010–2011 to 2023–2024 seasons: K163 to Q or others (E/N/R/T/I/L/H), N129 to D or others (E/K/G/S/Y/T/I/F), K130 to N or others (R/S/H/E/Q/I/T/D), and D127 to E or others (N/G/A). Infrequent D127E/N/G/A substitutions occurred in each season (0.08%–0.8%). The transition times in years were defined by the number of years from the first appearance of a mutation in the sample to its first reaching near fixation (>90%) in the viral population. B, Evolutions of epitope-specific HAI-Ab-IDs in post-sera and in donors (including pre-sera, post-sera, and pre- and post-sera) from 2010 to 2016. Abbreviations: HA, hemagglutinin; HAI-Ab-ID, hemagglutination inhibition antibody immunodominance; post-sera, postvaccination sera; pre-sera, prevaccination sera.
We next determined the evolution of these HAI-Ab-IDs from 2010 to 2016 (Figure 2B, Supplementary Table 1). We found that the highest proportions of K163-Ab-IDs and D127/N129/K130-Ab-IDs were present in the 2010 post-sera of donors and gradually decreased from 2010 to 2016. In contrast, D127/N129-Ab-ID was not observed in 2010 but gradually increased from 2011 to 2016. Interestingly, K130-Ab-IDs were present in higher proportions in pre-sera than post-sera, opposite to K163-Ab-IDs, D127/N129/K130-Ab-IDs, and D127/N129-Ab-IDs (Figure 1A). Our previous study and current data suggested that the preexisting HAI-Ab-IDs may drive HAI-Ab evolution by Ab-mediated epitope masking [].
K163-Ab-IDs in Adults Primed by USSR Group Viruses
Thirty participants (BYR, 1961–1983; median birth year [MBY], 1977) displayed K163-Ab-IDs (Figures 1A and 3A). To analyze CA/09-like vaccine-induced HAI-Ab populations, we performed HAI assays with 4 RG viruses and 12 IAVs circulating from 1918 to 2018 (Table 1, Figure 3, Supplementary Table 2). Thirty K163-Ab-ID participants were first divided into 3 groups based on prevaccination anti-RG-wt HAI-Ab titers: ≤20 (G1 = 23), 80 (G2 = 4), and 160–320 (G3 = 3). To determine the intensity of K163-Ab-IDs and the levels of non-K163-Abs, pre- and post-sera with anti-RG-wt HAI-Ab titers ≥40 (called positive pre- and post-sera, n = 37) were divided into 3 subgroups based on anti-RG-K163Q HAI-Ab titers: ≤10 (a = 17; ∼100% K163-Abs), 20 (b = 5), and ≥40 (c = 15; dominant K163-Abs mixed with significant non-K163-Abs [HAI-Ab titers ≥40]).
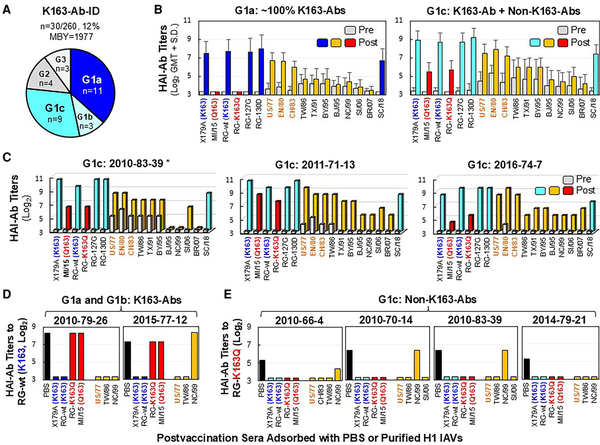
Figure 3
Immunization with CA/09-like vaccine elicited K163-Ab-IDs in some 1977–1983 middle-aged adults who were virus primed. Pre- and post-sera from 30 adults possessing K163-Ab-IDs were tested by HAI assays with the following: X179A vaccine strain possessing K163, A/Michigan/45/2015 field strain (MI/15c) containing Q163, RG-wt containing K163, RG-K163Q, RG-127G, RG-130D, 10 historical 1977–2007 A(H1N1) IAVs (A/USSR/90/1977 [US/77], A/England/333/1980 [EN/80], A/Chile/1/1983 [CH/83], A/Taiwan/1/1986 [TW/86], A/Texas/36/1991 [TX/91], A/Bayern/7/1995 [BY/95], A/Beijing/262/1995 [BJ/95], A/New Caledonia/20/1999 [NC/99], A/Solomon Islands/03/2006 [SI/06], A/Brisbane/59/2007 [BR/07]), and A/South Carolina/1/1918-TX/91 (SC/18). USSR group viruses (US/77, EN/80, and CH/83) as priming viruses are highlighted in orange. A, Three groups of adults primed with USSR group viruses (n = 30) who possessed various titers of preexisting anti-RG-wt HAI-Abs (G1 ≤20, G2 = 80, and G3 = 160–320) and 3 subgroups of G1 adults (n = 23) possessing no anti-RG-K163Q HAI-Ab in post-sera (G1a: anti-RG-K163Q titer ≤10, n = 11) or various titers of anti-RG-K163Q HAI-Abs of 20 (G1b, n = 3) and ≥40 (G1c, n = 9). B, HAI-Ab responses of G1a (∼100% K163-Abs) and G1c (dominant K163-Abs mixed with significant non-K163-Abs [anti-RG-K163Q HAI-Ab titers ≥40]). C, HAI-Ab responses of 3 G1c individuals exhibiting ≥4-fold HAI-Ab rises against 7 or 10 historical 1977–2007 A(H1N1) IAVs. D, Anti-RG-wt HAI-Ab titers in post-sera of G1a and G1b individuals after adsorptions with PBS control or 7 indicated purified H1 viruses. E, Anti-RG-K163Q HAI-Ab titers in post-sera of 4 G1c individuals after adsorptions with PBS control or 7 or 8 indicated purified H1 viruses. *Donor number is given by immunization year–birth year–testing number. Abbreviations: Ab, antibody; HAI, hemagglutination inhibition; IAV, influenza A virus; ID, immunodominance; MBY, median birth year; PBS, phosphate-buffered saline; post-sera, postvaccination sera; pre-sera, prevaccination sera; RG, reverse genetics.
G1a donors (n = 11) displayed extreme vaccine-induced K163-Ab-IDs (Figure 3A and 3B, Supplementary Table 2). The immunization resulted in ≥8-fold HAI-Ab rises against RG-wt (GMT, 22-fold) but no rise against RG-K163Q, and postvaccination HAI-Ab titers were 80 to 1280 against RG-wt but ≤10 against RG-K163Q. Such K163-Abs did not cross-inhibit with MI/15c (K163Q) field strain and the 1986–2007 strains containing the N125/T127 glycosylation motif, but they cross-inhibited well with SC/18, RG-130D, RG-127G (D127N/N129T), and USSR group viruses (US/77, EN/80, and CH/83) possessing an N127/T129 glycosylation motif.
G1c donors (n = 9) exhibited ≥4-fold HAI-Ab rises against RG-wt (GMT, 40-fold) and RG-K163Q (GMT, 5-fold), suggesting that the immunization simultaneously induced dominant K163-Abs and subdominant non-K163-Abs (defined by anti-RG-K163Q titers ≥40; Figure 3A and 3B, Supplementary Table 2). Non-K163-Abs cross-inhibited MI/15c well. Three G1c donors also showed ≥4-fold HAI-Ab rises against TW group viruses (TW/86, TX/91, and BY/95) and 1 or 4 NC group viruses (BJ/95, NC/99, SI/06, and BR/07) possessing HA 130 deletion (Figure 3C). Ab adsorption data showed that K163-Abs in G1a and G1b donors can be removed by US/77 + TW/86 ± NC/99 but not by RG-K163Q and MI/15e (Figure 3D). However, non-K163-Abs in 4 G1c donors can effectively be removed by RG-K163Q, MI/15e, and 3 historical 1977–2006 IAVs tested (Figure 3E). These data indicated that K163-Abs and most, if not all, non-K163-Abs in G1 donors (prevaccination anti-RG-wt HAI-Ab titers ≤20) were produced from MBCs.
Interestingly, in 4 G2 donors possessing extreme preexisting K163-Ab-IDs (titer = 80), the immunization mainly induced non-K163-Ab responses (Supplementary Table 2). These non-K163-Abs in G2 donors were not cross-inhibiting with 1977–2007 IAVs, suggesting that they may have been generated from naive B cells [, ]. Therefore, preexisting K163-Ab-IDs drove Ab evolution from MBC-derived cross-reactive K163-Abs in adults primed with USSR virus to naive B-cell–derived strain-specific non-K163-Abs, probably due to preexisting K163-Ab–mediated epitope masking [, , ].
D127/N129/K130-Ab-IDs in Adults Primed by TW Group Viruses
Forty-seven donors (BYR, 1967–1993; MBY, 1984) displayed D127/N129/K130-Ab-IDs (Figures 1A and 4A). To understand the events that promote development of such D127/N129/K130-Ab-IDs, we investigated the sera in HAI assays with 17 A(H1N1) IAVs (Table 1, Figure 4, Supplementary Table 3). Forty-seven participants were first divided into 4 groups based on prevaccination anti-RG-wt HAI-Ab titers: ≤10 (G1 = 13), 20 (G2 = 7), 40–80 (G3 = 16), and 160–1280 (G4 = 11). To determine the strengths of D127/N129/K130-Ab-IDs and the levels of non-D127/N129/K130-Abs, positive pre- and post-sera (n = 74) were divided into 3 subgroups based on anti-RG-130D HAI-Ab titers: ≤10 (a = 31; ∼100% D127/N129/K130-Abs), 20 (b = 13), and ≥40 (c = 30; dominant D127/N129/K130-Abs mixed with significant non-D127/N129/K130-Abs). As shown in Supplementary Table 3, 49% (23/47) of donors displayed extreme D127/N129/K130-Ab-IDs (anti-RG-wt titers = 40–640 and anti-RG-130D titers ≤10) in pre-sera (a = 7), post-sera (a = 8), or pre- and post-sera (a = 8). Most of the donors, who displayed extreme vaccine-induced D127/N129/K130-Ab-IDs, were primed with TW group viruses from 1986 to 1998 (Table 1). These D127/N129/K130-Abs cross-inhibited well against RG-K163Q, MI/15c (D127/N129/K130/Q163), and 1986–1995 TW group viruses possessing the N125/T127 glycosylation motif but cross-inhibited poorly against SC/18 (E127/T129/K130) and the 1977–1983 USSR group viruses possessing the N127/T129 glycosylation motif (Figure 4). The lack of HA recognition of 1977–1983 strains could be due to glycan-mediated shielding of the epitope [].
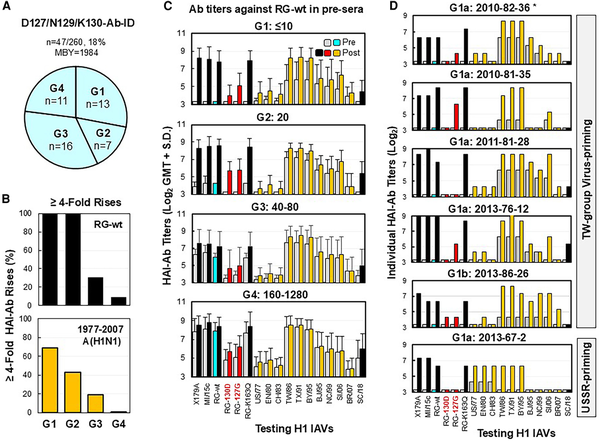
Figure 4
Immunization with CA/09-like vaccine elicited D127/N129/K130-Ab-IDs in some adults. Pre- and post-sera from 47 adults possessing D127/N129/K130-Ab-ID were tested by HAI assays with the following: X179A vaccine strain, A/Michigan/45/2015 field strain (MI/15c), RG-wt, RG-K163Q, RG-127G, RG-130D, 10 historical 1977–2007 A(H1N1) viruses (A/USSR/90/1977 [US/77], A/England/333/1980 [EN/80], A/Chile/1/1983 [CH/83], A/Taiwan/1/1986 [TW/86], A/Texas/36/1991 [TX/91], A/Bayern/7/1995 [BY/95], A/Beijing/262/1995 [BJ/95], A/New Caledonia/20/1999 [NC/99], A/Solomon Islands/03/2006 [SI/06], A/Brisbane/59/2007 [BR/07]), and A/South Carolina/1/1918-TX/91 (SC/18). A, Four groups of adults possessing various titers of preexisting anti-RG-wt HAI-Abs (G1 ≤10, G2 = 20, G3 = 40–80, and G4 = 160–1280). B, HAI-Ab rises (≥4-fold; percentage) against RG-wt or at least two 1977–2007 A(H1N1) viruses among 4 groups. C, HAI-Ab responses of the 4 groups. D, HAI-Ab responses of 6 G1 individuals with anti-RG-130D HAI titers: ≤10 (a) or 20 (b). *Donor number is given by immunization year–birth year–testing number. Abbreviations: Ab, antibody; HAI, hemagglutination inhibition; ID, immunodominance; MBY, median birth year; post-sera, postvaccination sera; pre-sera, prevaccination sera; RG, reverse genetics; wt, wild type.
Twenty-six (26/47, 55%) donors exhibited ≥4-fold HAI-Ab rises against X179A and RG-wt (Supplementary Table 3). Among the 26 donors, 15 (58%) also displayed ≥4-fold HAI-Ab rises against three to seven 1977–2007 A(H1N1) IAVs; the other 11 (42%) donors, who did not exhibit ≥4-fold HAI-Ab rises to 1977–2007 IAVs, possessed significant preexisting HAI-Abs (titers = 40–1280) specific for these historical viruses. Nevertheless, a correlation of fold rises of HAI-Ab responses against RG-wt and some 1977–2007 IAVs were observed among 4 groups (Figure 4B and 4C). Moreover, Ab adsorption data from 5 donors showed that D127/N129/K130-Abs can be effectively removed by TW viruses with or without other 1977–2007 A(H1N1) strains tested (Supplementary Figures 1 and 2). These data suggested that D127/N129/K130-Abs were generated from MBCs [].
Most donors (41/47, 87%; BYR, 1976–1993; MBY, 1985) exhibited TW virus–primed landscapes (defined as >4-fold higher HAI-Ab titers against TW viruses vs USSR viruses), while a few donors (6/47, 13%; BYR, 1967–1983; MBY, 1970) exhibited USSR virus–primed landscapes (Figure 4D, Supplementary Table 3). In the 1977–1978 US influenza season, only 0.4% of US/77-like isolates were recovered from patients ≤5 years old [], and the last epidemic of USSR group viruses occurred in the 1983–1984 season [], which suggested that most children born after 1976 were likely first infected with TW viruses but not USSR viruses. Our data demonstrate that most adults (41/42, 98%) born from 1976 to 1993 were likely primed by TW viruses, and they had D127/N129/K130-Ab-IDs from MBCs.
HAI-Ab Landscapes in Donors Possessing K130-Ab-IDs
Twenty-seven participants (BYR, 1963–1991; MBY, 1978) displayed K130-Ab-IDs (Figures 1A and 5A). To understand the possible mechanism that led the development of such K130-Ab-IDs, we performed HAI assays with 17 A(H1N1) IAVs (Table 1, Figure 5, Supplementary Table 4). Twenty-seven participants were first divided into 3 groups based on prevaccination anti-RG-wt HAI-Ab titers: ≤10 (G1 = 6), 40 (G2 = 13), and 80–320 (G3 = 8). To determine the levels of K130-Ab-IDs and non-K130-Abs, positive pre- and post-sera (n = 48) were divided into 3 subgroups based on anti-RG-130D HAI-Ab titers: ≤10 (a = 19; ∼100% K130-Abs), 20 (b = 8), and ≥40 (c = 21; dominant K130-Abs mixed with significant non-K130-Abs). As shown in Supplementary Table 4, 16 (16/27, 59%) adults displayed extreme K130-Ab-ID (anti-RG-wt titers = 40–320; anti-RG-130D titers ≤10) in pre-sera (a = 11), post-sera (a = 2), or pre- and post-sera (a = 3). Only 2 donors in G1a displayed extreme vaccine-induced K130-Ab-ID (anti-RG-wt titers = 40; anti-RG-130D titer ≤10). Nevertheless, K130-Abs cross-inhibited well with RG-K163Q, RG-127G, and MI/15c (Figure 5C and 5D).
Figure 5
Immunization with CA/09-like vaccine elicited K130-Ab-IDs likely targeting the receptor binding site. Pre- and post-sera from 27 adults possessing K130-Ab-ID were tested by HAI assays with the following: X179A vaccine strain, A/Michigan/45/2015 field strain (MI/15c), RG-wt, RG-K163Q, RG-127G, RG-130D, 10 historic 1977–2007 A(H1N1) viruses (A/USSR/90/1977 [US/77], A/England/333/1980 [EN/80], A/Chile/1/1983 [CH/83], A/Taiwan/1/1986 [TW/86], A/Texas/36/1991 [TX/91], A/Bayern/7/1995 [BY/95], A/Beijing/262/1995 [BJ/95], A/New Caledonia/20/1999 [NC/99], A/Solomon Islands/03/2006 [SI/06], and A/Brisbane/59/2007 [BR/07]), and A/South Carolina/1/1918-TX/91 (SC/18). A, Three groups of adults possessing various titers of preexisting anti-RG-wt HAI-Abs (G1 ≤10, G2 = 40, and G3 = 80–320). B, HAI-Ab rises (≥4-fold; percentage) against RG-wt or at least two 1977–2007 A(H1N1) viruses among 3 groups. C, HAI-Ab responses of the 3 groups. D, HAI-Ab responses of 6 G1 individuals with anti-RG-130D HAI titers: ≤10 (a) or ≥40 (c). *Donor number is given by immunization year–birth year–testing number. Abbreviations: Ab, antibody; HAI, hemagglutination inhibition; ID, immunodominance; MBY, median birth year; post-sera, postvaccination sera; pre-sera, prevaccination sera; RG, reverse genetics; wt, wild type.
A correlation of fold rises of HAI-Abs against RG-wt and some historic 1977–2007 A(H1N1) IAVs was observed among the 3 groups (Figure 5B and 5C). Notably, all G1 donors (n = 6) displayed ≥4-fold HAI-Ab rises against RG-wt and multiple 1977–2006 IAVs (Figure 5D, Supplementary Table 4). Three G1 donors (BYR, 1984–1991) displayed a TW virus–primed pattern, and another 3 G1 donors (BYR, 1963–1969) exhibited a USSR virus–primed pattern. Interestingly, each G1 donor showed a unique HAI-Ab landscape. Moreover, unique HAI-Ab landscapes were observed in 3 G2 donors who showed ≥4-fold HAI-Ab rises against multiple 1977–2006 IAVs. Such highly unique HAI-Ab landscapes suggested that K130-Abs in different donors arose from MBCs with distinct cross-reactivity [].
D127/N129-Ab-ID in Adults Primed by TW Group or NC Group Viruses
Twenty-seven participants (BYR, 1980–1998; MBY, 1989) displayed D127/N129-Ab-ID (Figures 1A and 6A). They were investigated in HAI assays with 17 A(H1N1) IAVs (Table 1, Figure 6, Supplementary Table 5). Twenty-seven donors were first divided into 3 groups based on prevaccination anti-RG-wt HAI-Ab titers: ≤20 (G1 = 8), 40 (G2 = 6), and 80 to 320 (G3 = 13). To determine the levels of D127/N129-Ab-ID and non-D127/N129-Abs, positive pre- and post-sera (n = 46) were divided into 3 subgroups based on anti-RG-127G HAI-Ab titers: ≤10 (a = 11; ∼100% D127/N129-Abs), 20 (b = 14), and ≥40 (c = 21; dominant D127/N129-Abs mixed with significant non-D127/N129-Abs). Ten (10/27, 37%) individuals showed extreme D127/N129-Ab-ID (anti-RG-wt titers = 40–80, anti-RG-127G titers ≤10) in pre-sera (a = 7), post-sera (a = 2), or pre- and post-sera (a = 1). These D127/N129-Abs cross-inhibited well with RG-K163Q, RG-130D, and MI/15c but cross-inhibited poorly with SC/18 (E127/T129; Figure 6C and 6D , Supplementary Table 5).
Figure 6
Immunization with CA/09-like vaccine elicited strain-specific D127/N129-Ab-ID in some young adults. Pre- and post-sera from 27 adults possessing D127/N129-Ab-ID were tested by HAI assays with the following: X179A vaccine strain, A/Michigan/45/2015 field strain (MI/15c), RG-wt, RG-K163Q, RG-127G, RG-130D, 10 historical 1977–2007 A(H1N1) viruses (A/USSR/90/1977 [US/77], A/England/333/1980 [EN/80], A/Chile/1/1983 [CH/83], A/Taiwan/1/1986 [TW/86], A/Texas/36/1991 [TX/91], A/Bayern/7/1995 [BY/95], A/Beijing/262/1995 [BJ/95], A/New Caledonia/20/1999 [NC/99], A/Solomon Islands/03/2006 [SI/06], A/Brisbane/59/2007 [BR/07]), and A/South Carolina/1/1918-TX/91 (SC/18). A, Three groups of adults possessing various titers of preexisting anti-RG-wt HAI-Abs (G1 ≤20, G2 = 40, G3 = 80–320). B, HAI-Ab rises (≥4-fold; percentage) against RG-wt or at least two 1977–2007 A(H1N1) viruses among 3 groups. C, HAI-Ab responses of 3 groups. D, HAI-Ab responses of 6 G1 individuals with anti-RG-127G HAI titers: ≤10 (a), 20 (b), or ≥40 (c). *Donor number is given by immunization year–birth year–testing number. Abbreviations: Ab, antibody; HAI, hemagglutination inhibition; ID, immunodominance; MBY, median birth year; post-sera, postvaccination sera; pre-sera, prevaccination sera; RG, reverse genetics; wt, wild type.
Data from Ab landscapes and birth years indicated that most donors (20/27, 74%; BYR, 1980–1995) were likely primed with TW viruses and a few donors (5/27, 19%; BYR, 1992–1998) were likely primed with NC viruses. Notably, only 1 donor (2015-90-48) showed 4-fold HAI-Ab rises against TW viruses. Ab adsorption data showed that donor 2015-90-48 contained 2 vaccine-induced HAI-Ab populations—strain-specific Ab and cross-reactive Ab against RG-wt and TW/86 virus—while another donor (2015-81-22) contained only strain-specific Ab (Supplementary Figure 3). These data indicated that D127/N129-Abs were CA/09 strain specific and not generated from 1986–2007 A(H1N1)-derived MBCs.
DISCUSSION
Understanding the cross-reactive and strain-specific epitopes on influenza viruses among humans with or without preexisting immunity will focus efforts to forecast influenza virus antigenic drift and aid influenza vaccine stain selection [, , ]. We assessed HAI-Ab-IDs for A(H1N1)pdm09 IAVs in adults before and after immunization from 2010 to 2016 (Figures 1 and 2, Supplementary Table 1). During this time, 71% to 42% adults exhibited HAI-Ab-IDs. As most adults had little to no preexisting HAI-Abs against CA/09-like viruses in 2010, these adults recalled MBCs against conserved epitopes containing K163, D127/N129/K130, or K130 to produce HAI-Ab-IDs cross-inhibiting well with their priming historical A(H1N1) IAVs (Figures 3–5, Supplementary Tables 2–4, Supplementary Figures 1–2) [, , , ]. Conversely, the majority of adults in 2016 possessed HAI-Ab-IDs targeting a new D127/N129-epitope. D127/N129-Abs showed no cross-inhibition with 1977–2007 A(H1N1) IAVs (Figure 6, Supplementary Table 5, Supplementary Figure 3), suggesting that they were generated from naive B cells [, ]. Therefore, shifts of MBC-derived cross-reactive HAI-Ab-IDs to naive B-cell–derived strain-specific HAI-Ab-ID occurred between 2010 and 2016 (Figure 2B).
HAI-Ab-IDs were observed before immunization between 2010 (26%) and 2016 (32%; Figure 1, Supplementary Tables 1–5). Our previous study demonstrated that preexisting K163-Ab-IDs masked the K163 epitope and redirected a CA/09-like vaccine-induced HAI-Ab response to a strain-specific epitope in adults primed with USSR virus [, ]. However, whether preexisting D127/N129/K130-Ab-IDs, K130-Ab-IDs, and D127/N129-Ab-ID in some adults could also redirect the HAI-Ab response through Ab-mediated epitope masking is unknown. From 2010 to 2016, HAI-Ab-IDs targeting cross-reactive epitopes gradually decreased while HAI-Ab-IDs targeting strain-specific epitopes increased (Figure 2), indicating that circulating HAI-Ab-IDs evolved with viral antigenic drift.
HAI-Ab-IDs drove A(H1N1)pdm09 viral evolution by HAI-Ab–mediated immune selection. Since the 2010–2011 season, infrequent escape mutants at HA positions of K163, D127, N129, or K130 have been detected in clinic samples (in vivo selection; Figure 2). These escape mutants were also selected in vitro by human monoclonal antibodies [, , ] or polyclonal antisera possessing HAI-Ab-ID [, ]. Such HAI-Ab–mediated selection eventually resulted in multiple escape mutants at each position, until variants possessing antigenic novelty and high fitness for infectivity, replication ability, and transmissibility finally became fixed in the human population. For example, the K163Q variant—which possessed the greatest antigenic drift as compared with other K163 mutants [], better competitive growth vs wild type virus [], and good structure fitness []—became fixed in the 2013–2014 season []. TT from the first appearance of a K163Q mutant to its fixation was 4 years, which is the shortest TT in the record []. The N129D mutation first emerged in the 2010–2011 season and became fixed in the 2020–2021 season (TT = 11 years). This change increased receptor binding affinity and viral replication but had only a moderate impact on antigenicity [, , ]. Conversely, the K130N mutation in CA/09-like virus background significantly changed antigenicity but also decreased receptor binding affinity []. The K130N variant followed by the N129D change became fixed in the 2022–2023 season (TT = 13 years). Strong selection of the D127E mutation was observed in vitro with monoclonal antibody EM4C04, which was isolated from an adult with CA/09-like virus infection who was likely primed by TX/91-like virus [, , ]. However, the D127E mutation increased the receptor binding ability for α(2,3)-Gal-linked SA [], which may have decreased transmissibility in humans []. This may explain why D127 variants were infrequently detected among circulating A(H1N1)pdm09 IAVs from 2010 to 2024 (Figure 2).
K130 deletion potentially reduced the size of the RBS pocket, thus preventing RBS-targeted Abs from inserting their long heavy-chain complementarity-determining region 3 into the pocket [, , , ]. The RBS-targeted Abs have a small binding footprint [, ]; N-glycosylation at HA 127/129 cannot block the binding [, ]. Therefore, we assume that most K130-Abs were the RBS-targeted Abs. Interestingly, K130-Ab-IDs mainly presented in pre-sera (Figure 1A, Supplementary Table 4). Our previous study demonstrated that most K130-Ab-IDs in adults with CA/09-like virus infection who were critically ill cross-inhibited with A(H3N2) or influenza B viruses (IBVs) []. Thus, we infer that some K130-Abs in pre-sera may be induced by infections with A(H3N2) or IBVs in previous season. Whether K130-Ab-IDs were associated with priming with A(H3N2) or IBVs is unknown.
There are several limitations in this study. First, only 4 RG viruses and adult sera were used; more RG viruses covering different antigenic sites and more sera from different age cohorts should be considered in future studies. Second, each HAI-Ab-ID can select multiple amino acids in a single HA position; which amino acid and why it became fixed was not investigated here. Third, the functional and biophysical constraints that govern viral evolution and TTs were not investigated. Finally, D127/N129/K130 epitope and D127/N129 epitope can be shielded by a D127N/N129T glycosylation site (Figures 4 and 6). Although modeling of a D127N/N129T glycosylation site was completed in Glycam software [], whether this glycosylation site could effectively shield the same amino acids for the 2 epitopes needs to be further assessed. Notably, HAI-Abs targeting the epitope possessing D127 and 150 loop were observed in infants first infected with CA/09-like virus []. Whether the increased numbers of adults possessing strain-specific D127/N129-Ab-ID led to the emergence of the recent variant containing N129D/K130N/N156K mutations is under investigation. Building a pipeline for data-driven predictive analysis of viral and neutralizing Ab coevolution is important for influenza vaccine strain selection []. Data published in this study provide a foundation for future study of coevolution of neutralizing Abs and seasonal A(H1N1)pdm09 virus.
Notes
Acknowledgments . We gratefully acknowledge the authors from the originating and submitting laboratories of the sequences from GISAID's EpiFlu Database. We thank the Influenza Genomics and Diagnostic Team in the Virology Surveillance and Diagnosis Branch, Influenza Division, Centers for Disease Control and Prevention, for sequencing assistance.
Disclaimer . The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention and the funding agency.
Financial support. This work was supported by the Centers for Disease Control and Prevention.
References
- 1. Uyeki TM, Hui DS, Zambon M, Wentworth DE, Monto AS. Influenza. Lancet 2022; 400:693–706.
- 2. Thompson WW, Shay DK, Weintraub E, et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86.
- 3. Garten RJ, Davis CT, Russell CA, et al Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201.
- 4. Tumpey TM, Basler CF, Aguilar PV, et al Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005; 310:77–80.
- 5. Petrova VN, Russell CA. The evolution of seasonal influenza viruses. Nat Rev Microbiol 2018; 16:47–60.
- 6. Wu NC, Wilson IA. A perspective on the structural and functional constraints for immune evasion: insights from influenza virus. J Mol Biol 2017; 429:2694–709.
- 7. Guthmiller JJ, Utset HA, Wilson PC. B cell responses against influenza viruses: short-lived humoral immunity against a life-long threat. Viruses 2021; 13:965.
- 8. Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011; 9:669–83.
- 9. Angeletti D, Yewdell JW. Is it possible to develop a “universal” influenza virus vaccine? Outflanking antibody immunodominance on the road to universal influenza vaccination. Cold Spring Harb Perspect Biol 2018; 10:a028852
- 10. Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat Rev Immunol 2019; 19:383–97.
- 11. Raymond FL, Caton AJ, Cox NJ, Kendal AP, Brownlee GG. The antigenicity and evolution of influenza H1 haemagglutinin, from 1950–1957 and 1977–1983: two pathways from one gene. Virology 1986; 148:275–87.
- 12. Kendal AP, Joseph JM, Kobayashi G, et al Laboratory-based surveillance of influenza virus in the United States during the winter of 1977–1978. I: periods of prevalence of H1N1 and H3N2 influenza A strains, their relative rates of isolation in different age groups, and detection of antigenic variants. Am J Epidemiol 1979; 110:449–61.
- 13. Cox NJ, Black RA, Kendal AP. Pathways of evolution of influenza A (H1N1) viruses from 1977 to 1986 as determined by oligonucleotide mapping and sequencing studies. J Gen Virol 1989; 70(pt 2):299–313.
- 14. Xu X, Rocha EP, Regenery HL, Kendal AP, Cox NJ. Genetic and antigenic analyses of influenza A (H1N1) viruses, 1986–1991. Virus Res 1993; 28:37–55.
- 15. McDonald NJ, Smith CB, Cox NJ. Antigenic drift in the evolution of H1N1 influenza A viruses resulting from deletion of a single amino acid in the haemagglutinin gene. J Gen Virol 2007; 88:3209–13.
- 16. Daum LT, Canas LC, Smith CB, et al Genetic and antigenic analysis of the first A/New Caledonia/20/99-like H1N1 influenza isolates reported in the Americas. Emerg Infect Dis 2002; 8:408–12.
- 17. Hancock K, Veguilla V, Lu X, et al Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–52.
- 18. Linderman SL, Chambers BS, Zost SJ, et al Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proc Natl Acad Sci U S A 2014; 111:15798–803.
- 19. Li Y, Myers JL, Bostick DL, et al Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J Exp Med 2013; 210:1493–500.
- 20. Lu X, Guo Z, Li ZN, et al Low quality antibody responses in critically ill patients hospitalized with pandemic influenza A(H1N1)pdm09 virus infection. Sci Rep 2022; 12:14971.
- 21. Francis T Jr, Davenport FM, Hennessy AV. A serological recapitulation of human infection with different strains of influenza virus. Trans Assoc Am Physicians 1953; 66:231–9.
- 22. Raymond DD, Bajic G, Ferdman J, et al Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc Natl Acad Sci U S A 2018; 115:168–73.
- 23. Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Basler CF, Crowe JE. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J Virol 2011; 85:10905–8.
- 24. Hong M, Lee PS, Hoffman RM, et al Antibody recognition of the pandemic H1N1 influenza virus hemagglutinin receptor binding site. J Virol 2013; 87:12471–80.
- 25. Krause JC, Tsibane T, Tumpey TM, et al Epitope-specific human influenza antibody repertoires diversify by B cell intraclonal sequence divergence and interclonal convergence. J Immunol 2011; 187:3704–11.
- 26. O’Donnell CD, Vogel L, Wright A, et al Antibody pressure by a human monoclonal antibody targeting the 2009 pandemic H1N1 virus hemagglutinin drives the emergence of a virus with increased virulence in mice. mBio 2012; 3:e00120-12.
- 27. Wrammert J, Koutsonanos D, Li GM, et al Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 2011; 208:181–93.
- 28. Liu F, Tzeng WP, Horner L, et al Influence of immune priming and egg adaptation in the vaccine on antibody responses to circulating A(H1N1)pdm09 viruses after influenza vaccination in adults. J Infect Dis 2018; 218:1571–81.
- 29. Huang KY, Rijal P, Schimanski L, et al Focused antibody response to influenza linked to antigenic drift. J Clin Invest 2015; 125:2631–45.
- 30. Zost SJ, Wu NC, Hensley SE, Wilson IA. Immunodominance and antigenic variation of influenza virus hemagglutinin: implications for design of universal vaccine immunogens. J Infect Dis 2019; 219:S38–45.
- 31. Lu X, Liu F, Tzeng WP, York IA, Tumpey TM, Levine MZ. Antibody-mediated suppression regulates the humoral immune response to influenza vaccination in humans. J Infect Dis 2024; 229:310–21.
- 32. Altman MO, Angeletti D, Yewdell JW. Antibody immunodominance: the key to understanding influenza virus antigenic drift. Viral Immunol 2018; 31:142–9.
- 33. Davis AKF, McCormick K, Gumina ME, et al Sera from individuals with narrowly focused influenza virus antibodies rapidly select viral escape mutations in ovo. J Virol 2018; 92:e00859-18.
- 34. Ji W, Guthmiller J. Goldilocks zone of preexisting immunity: too little or too much suppresses diverse antibody responses against influenza viruses. J Infect Dis 2024; 229:299–302.
- 35. Cox NJ, Kendal AP. Genetic stability of A/Ann Arbor/6/60 cold-mutant (temperature-sensitive) live influenza virus genes: analysis by oligonucleotide mapping of recombinant vaccine strains before and after replication in volunteers. J Infect Dis 1984; 149:194–200.
- 36. Turner JS, Zhou JQ, Han J, et al Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 2020; 586:127–32.
- 37. Gouma S, Anderson EM, Hensley SE. Challenges of making effective influenza vaccines. Annu Rev Virol 2020; 7:495–512.
- 38. Guthmiller JJ, Han J, Li L, et al First exposure to the pandemic H1N1 virus induced broadly neutralizing antibodies targeting hemagglutinin head epitopes. Sci Transl Med 2021; 13:eabg4535.
- 39. Li GM, Chiu C, Wrammert J, et al Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012; 109:9047–52.
- 40. Yasuhara A, Yamayoshi S, Soni P, et al Diversity of antigenic mutants of influenza A(H1N1)pdm09 virus escaped from human monoclonal antibodies. Sci Rep 2017; 7:17735.
- 41. Li C, Hatta M, Burke DF, et al Selection of antigenically advanced variants of seasonal influenza viruses. Nat Microbiol 2016; 1:16058.
- 42. Shih AC, Hsiao TC, Ho MS, Li WH. Simultaneous amino acid substitutions at antigenic sites drive influenza A hemagglutinin evolution. Proc Natl Acad Sci U S A 2007; 104:6283–8.
- 43. Lee N, Khalenkov AM, Lugovtsev VY, et al The use of plant lectins to regulate H1N1 influenza A virus receptor binding activity. PLoS One 2018; 13:e0195525.
- 44. Lee CY, Raghunathan V, Caceres CJ, et al Epistasis reduces fitness costs of influenza A virus escape from stem-binding antibodies. Proc Natl Acad Sci U S A 2023; 120:e2208718120.
- 45. Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol 2021; 19:528–45.
- 46. Ekiert DC, Kashyap AK, Steel J, et al Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 2012; 489:526–32.
- 47. Koel BF, Mogling R, Chutinimitkul S, et al Identification of amino acid substitutions supporting antigenic change of influenza A(H1N1)pdm09 viruses. J Virol 2015; 89:3763–75.
- 48. Lyons DM, Lauring AS. Mutation and epistasis in influenza virus evolution. Viruses 2018; 10:407.
- 49. Meijers M, Ruchnewitz D, Eberhardt J, Karmakar M, Luksza M, Lassig M. Concepts and methods for predicting viral evolution. Methods Mol Biol 2025; 2890:253–90.
- 50. Han AX, de Jong SPJ, Russell CA. Co-evolution of immunity and seasonal influenza viruses. Nat Rev Microbiol 2023; 21:805–17.