INTRODUCTION
Over 40 years ago, Clostridioides difficile infection (CDI) was recognized as the cause of pseudomembranous colitis associated with antibiotic use []. Since this discovery, CDI has become the leading cause of gastroenteritis-associated death in the United States [, ] and the most commonly reported healthcare-associated pathogen []. The major pathophysiology of CDI is attributed to a dysbiosis in colonic microbiota, usually triggered by antibiotic use []. Early text on CDI noted that, endoscopically, the disease only appeared to affect the colon and did not cross the ileocecal valve []. Molecular receptor interaction between colonic enterocytes and C. difficile together with physiologic differences between the small intestine and colon (slow transit time) are postulated as contributing to colonic predominance in colonization and disease []. However, there is increasing evidence of small bowel disease termed C. difficile enteritis (CDE).
The first case report of CDE in 1980 described a patient with fever and increased ileostomy output []. Since this time, case reports of CDE have increased; prior to 2008, there were 25 cases in the literature, but 29 cases were reported during 2008–2010 []. The most recent systematic review noted 67 cases through July 2012, but it asserts that true prevalence is likely much higher []. Severe CDE, including fulminant enteritis, intestinal perforation, and fatal BI/NAP1/O27 disease have been reported []. C. difficile enteritis mortality rates are 32% in pooled analysis of case reports []. In patients who have had colectomy, CDE has been reported to occur up to 9 years after the procedure []. Along with cytomegalovirus infection, CDE also has been implicated as a trigger for chronic pouchitis after ileal pouch-anal anastomosis (IPAA) [].
The pathophysiology of CDE is not well understood. Molecular studies have identified that C. difficile has similar ability, in vitro, to bind both enterocytes and colonocytes []. This, together with C. difficile colonization in the small bowel of 3% of autopsy patients with nongastrointestinal disease, suggests a reservoir for eventual infection []. Furthermore, the terminal ileum undergoes colonic metaplasia after colectomy, [] and the microbiota more closely resemble that of colonic flora []. These changes, together with antibiotic alterations in flora, also are likely contributing factors to CDE.
The prevalence of inflammatory bowel disease (IBD; especially Crohn's disease) is increasing worldwide [, ], and, despite significant improvement in medical therapy, proctocolectomy with or without IPAA is still the treatment of choice for patients with medically refractory disease, high-grade dysplasia, or cancer []. Total colectomy is also the preferred management for the majority of patients with familial adenomatous polyposis []. All potential consequences—however infrequent—of total colectomy should be defined to improve patient-centered postcolectomy care. Therefore, our objective was to pragmatically evaluate CDE prevalence, severity, and outcomes using a large population of postcolectomy patients at an academic hospital. Additionally, we sought to discover potential CDE risk factors that could generate hypotheses regarding this rare infection.
METHODS
Study Design and Population
This study was approved by the University of Michigan's Institutional Review Board. We retrospectively reviewed electronic medical records (EMR) of all patients' aged ≥18 years who underwent total colectomy from January 1993 to February 2013. In this cohort, patients with positive C. difficile testing through the clinical microbiology lab were reviewed in further detail to determine if they met the study definition of CDE. Postcolectomy patients without C. difficile testing and those not meeting criteria for CDE served as the control group.
Data Collection and Study Definitions
For both the CDE and control group, all authors (except SC) were trained in data extraction; they manually abstracted the entire data set including demographics, baseline patient and surgical factors, and outcome measures from the EMR using a standardized collection form.
We defined CDE as positive C. difficile testing occurring after total colectomy in a patient with documented symptoms (abdominal pain, fever, diarrhea, or increased ostomy output), imaging findings (ileus, small bowel thickening, or edema), and/or pseudomembranes on small bowel enteroscopy. Prior to 2009, all C. difficile tests performed by our clinical microbiology laboratory were based on various single-step enzyme immunoassays (EIAs). From 2009 onward, all C. difficile tests were multistep: an initial EIA for both glutamate dehydrogenase (GDH) antigen and toxins A/B, followed by polymerase chain reaction (PCR) for the tcdB gene for GDH/toxin discordant results.
Patient characteristics, such as mechanical ventilation, proton pump inhibitor (PPI) use, total parenteral nutrition (TPN) and enteral feeding were evaluated over a time course spanning 30 days before surgery to 30 days after surgery. Antibiotic exposure was evaluated during the 30 days prior to surgery by reviewing preoperative outpatient notes, outpatient prescriptions entered, inpatient notes during admission for colectomy, and the inpatient medication administration record. Immunosuppression was defined as an innate immune system deficiency (AIDS) or exogenous causes of suppression (prednisone use of ≥5 mg per day or any steroid-sparing agent) at the time of surgery. In CDE cases, additional data collection included exposure to antibiotics and PPI during the 30 days prior to the CDE episode.
Surgical characteristics, such as the presence of ileoanal anastomosis with pouch formation or retention of a rectosigmoid (Hartmann) pouch was determined through detailed review of operative reports. Colectomy was considered emergent if the procedure was not an elective procedure planned prior to admission.
Regarding outcome measures, severe CDE was defined as having any of the following: leukocytosis (>15 000 white blood cells per µL), acute organ dysfunction (2-fold creatinine rise from baseline or urine output <0.5 mg/kg/hr for 12 hours per established criteria []; acute respiratory distress syndrome, either noted in the patient's chart or meeting criteria of a PaO2/FiO2 ≤200 and acute onset of diffuse infiltrates; new or worse heart failure; liver failure manifesting as new or worse coagulopathy and/or hepatic encephalopathy; shock with systolic blood pressure <90 and/or the need for vasopressors/inotropes), infection in an immunocompromised host or presence of sepsis (as previously defined []). Late-onset CDE was defined as occurring >90 days after colectomy, a cut-point chosen as C. difficile risk has been described to be highest in the months after antibiotic exposure []. Recurrent disease was defined as another episode of CDE meeting our specified criteria occurring >10 days after the index CDE case.
Statistical Analysis
All analyses were conducted in R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Data extracted through chart review and structured query of the EMR were cleaned, combined, and assessed for missing values and outliers. Following this, descriptive statistics were calculated including means and standard deviations for continuous variables and proportions for categorical variables. To assess if putative predictors were associated with CDE on unadjusted analysis, we used Cox proportional hazards regression model with CDE as the dependent variable (the coxph function from the R package survival [] was used). Patients were censored at death or the last noted clinical visit in the chart. On all analyses, P values less than .05 were considered statistically significant. We then assessed the significant variables visually for departures from the proportionality assumption. The final multivariable model was built in a stepwise fashion starting with the significant variables from unadjusted analysis and pruning using backward elimination and the likelihood ratio test with a cutoff of P < .05 for retention of variables. Sensitivity analysis for a specific variable was performed where relevant (see Results section) by comparing models with and without that variable, again by likelihood ratio testing. Among patients with CDE, we modeled risk factors for severity and recurrence using logistic regression and Cox proportional hazards, respectively.
RESULTS
CDE Frequency, Presenting Symptoms, and Severity
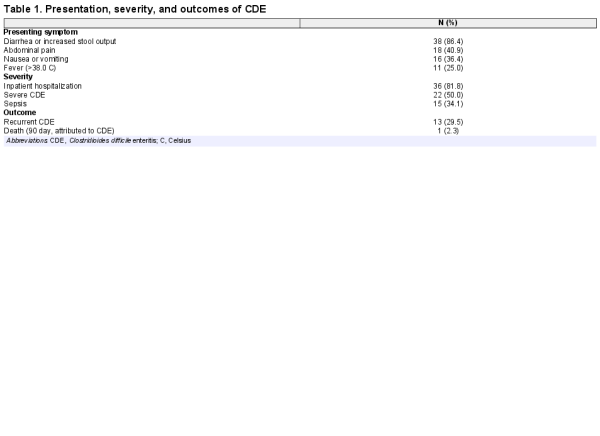
We identified 868 patients that had a total colectomy during the study period, of which 13 did not have data available for chart review, leaving a total study cohort of 855 patients. Our case definition of CDE was met in 44 of 855 (5.1%) postcolectomy patients. Clostridioides difficile enteritis occurred a median of 130 days (interquartile range 31–672 days) after colectomy, as illustrated in Figure 1. Nearly half (20 of 44) of CDE cases were diagnosed within 90 days of colectomy. Presenting symptoms, severity, and outcomes of CDE are in Table 1. The most common presenting symptom was diarrhea or increased stool output in 86.4% of cases. In half of the cases, CDE presented as severe disease, and 81.8% of CDE cases required inpatient admission.
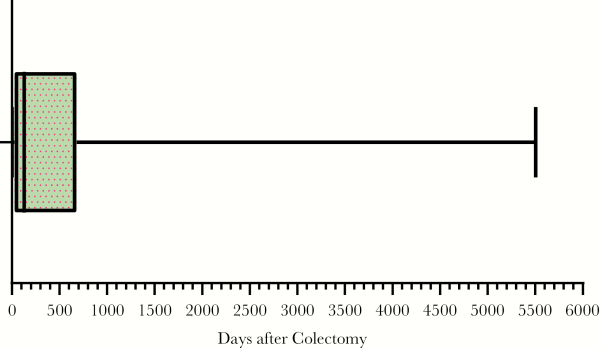
Figure 1
Time From Colectomy to Positive Clostridioides difficile Test
Baseline Patient and Surgical Characteristics
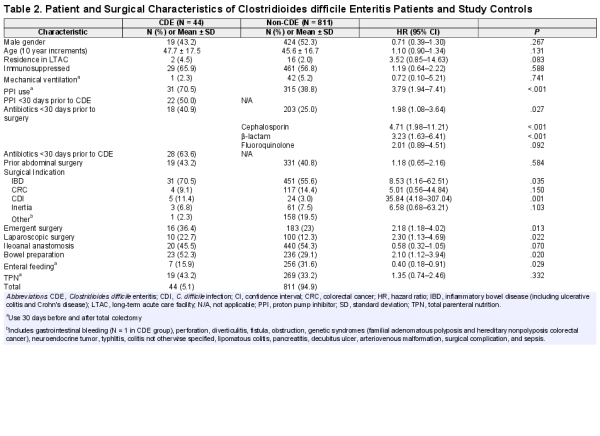
The baseline characteristics of CDE and control cases are outlined in Table 2. There were no statistically significant differences between the cases or controls with regards to age, gender, residence at a long-term facility, prior abdominal surgery, or ventilator status. In the unadjusted analysis, CDE was associated with antibiotics during the 30 days prior to surgery (hazard ratio [HR], 1.98; confidence interval [CI], 1.08–3.64; P = .027), most notably cephalosporin and β-lactam use. Proton pump inhibitor use was strongly associated with increased CDE risk (HR, 3.79; CI, 1.94–7.41; P ≤ .001), as illustrated in Figure 2. Surgical indications associated with higher CDE risk included CDI (HR, 35.84; CI, 4.18–307.04; P = .001) and IBD (HR, 8.53; CI, 1.16–62.51; P = .035). Emergent surgery (HR, 2.18; CI, 1.18–4.02; P = .013), bowel preparation (HR, 2.10; CI, 1.12–3.94; P = .020), and a laparoscopic procedure (HR, 2.30; CI, 1.13–4.69; P = .022) were other operative aspects associated with increased CDE risk. Enteral feeding (HR, 0.40; CI, 0.18–0.91; P = .029) was the only factor significantly associated with a decreased risk of CDE. In patients with late onset CDE (>90 days after colectomy), 11 of 24 (46%) received antibiotics and 10 of 24 (41.6%) received a PPI within the 30 days preceding the CDE episode.
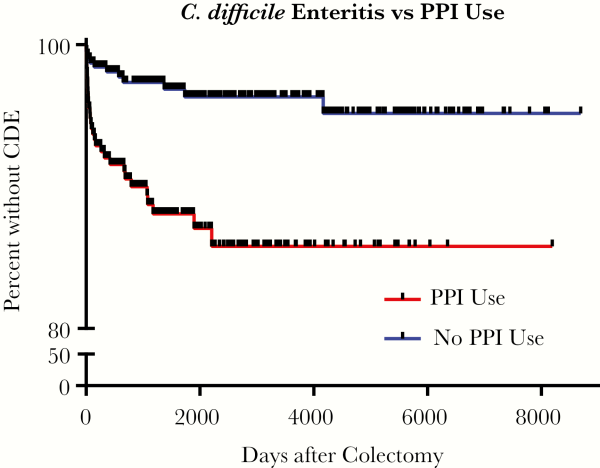
Figure 2
Time-to-Event Curve of Clostridioides difficile Enteritis Stratified by Type of Proton Pump Inhibitor Use
CDE Risk Factor Multivariable Model
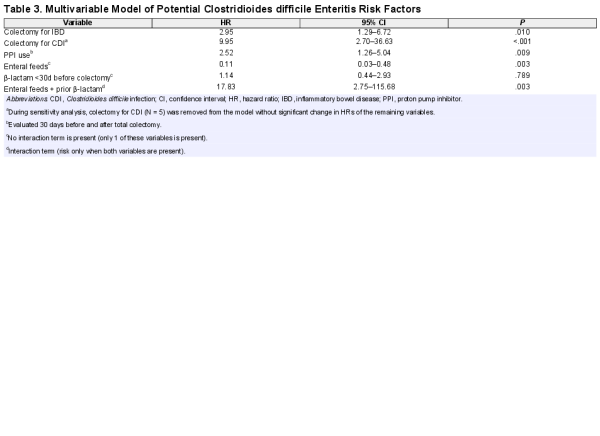
After adjustment for multiple variables (Table 3), undergoing colectomy for CDI or IBD and perioperative PPI use remained the most important CDE risk factors. Interestingly, prior β-lactam use did not meet significance unless it was present with enteral feeds, with a potential interaction greatly increasing the risk of CDE (HR, 17.83; CI, 2.75–115.68; P = .003). Interaction modeling suggests enteral feeds could be protection independently against CDE, but a possible interaction may amplify the risk of CDE when combined with β-lactams.
To address the possibility that positive C. difficile testing postcolectomy reflected bacterial backwash from prior CDI, we performed a sensitivity analysis using our final multivariable model, eliminating all cases whose indication for colectomy was CDI (N = 5). The remaining HR and CI were minimally changed, with no loss of statistical significance.
Severe and Recurrent CDE Risk Factors
CDE met criteria for severe CDE in half (22 of 44) of the cases. Factors associated with an increased risk for severe CDE using bivariable analysis included male gender (HR, 16.00; CI, 3.43–74.70; P ≤ .001) and PPI use (HR, 4.29; CI, 1.02–15.40; P = .025). Recurrent CDE occurred in 13 of 44 cases (29.5%), with a mean interval of 161 days (standard deviation ±138 days) between initial CDE and recurrent CDE. The main risk factor associated with recurrent CDE was antibiotic use within 30 days of initial CDE (HR, 4.90; CI, 1.08–22.18; P = .039). When recurrent CDE was defined to be more consistent with contemporary CDI criteria (>15 days), 1 case was removed from the analysis, but there was little effect on the results (HR, 4.83; CI, 1.04–22.47; P = .045).
DISCUSSION
C. difficile enteritis is a rare, but genuine, entity that should be on the differential diagnosis for postcolectomy patients presenting with fever, abdominal pain, and increased stool output. Our findings indicate CDE usually occurs within 1 year of colectomy, but it can be seen decades afterwards. It especially should be considered in patients with a colectomy indication of CDI or IBD, perioperative PPI use, and patients receiving both β-lactams and enteral feeds. It often (50%) presents with severe disease requiring hospitalization, and it has similar recurrence rates (29.5%) when compared to traditional CDI of the colon.
These findings represent the largest cohort study of CDE to date, finding 44 cases of CDE by examining 855 total colectomy patients over 20 years at a large, academic institution. Previous CDE case series have been unable to predict prevalence due to differences in study design [] or difficulty distinguishing clinical disease from colonization, which is critical as C. difficile toxin is present in up to 16% of postcolectomy patients at autopsy for nongastrointestinal causes [, ]. Using symptomatic disease as a requirement in our case definition, we found that CDE affects 5.1% of postcolectomy patients.
Our findings elaborate on previous research regarding CDE risk factors. Shen et al examined 21 cases of jejunal pouch CDI, concluding male gender was the strongest risk factor []. We found a similar gender preference for severe CDE, but there was no apparent gender preference using the total CDE cohort. Kim et al pooled 56 reported cases of CDE and described previous antibiotic use in 89% and IBD in 52% []. Our cohort was concurrent with these findings as antibiotics were common (64% of our cases) in the month prior to CDE, and our analysis suggests IBD as an indication for colectomy is a CDE risk factor. Tsiouris et al evaluated 310 patients who underwent total proctocolectomy between 2000 and 2009, finding a high prevalence of CDE (16%). The discrepancy between the prior prevalence estimate and ours (5.1%) may be driven by Tsiouris et al study period being concentrated during the CDI epidemic driven by the BI/NAP/027 strain, whereas our study included cases before and after the outbreak. Another possible explanation for the differences in prevalence could be an increasing emphasis on antibiotic stewardship during the later years of our study, which has been shown to decrease the risk of CDI []. Finally, C. dificile testing in postcolectomy patients was likely low during the earliest years of our study period due to decreased recognition of CDE as a clinical entity. Regarding CDE risk factors, Tsiouris et al suggested age and emergent surgery predisposed to CDE, whereas traditional CDI risk factors like prior antibiotic use were not significant. In our multivariable analysis, we did not find age or emergency surgery to be associated with CDE and we found antibiotic exposure may be less significant, with the notable exception of β-lactam use with enteral feeds.
When comparing our results to traditional CDI risk factors, there are remarkable similarities. The increased risk of CDI from PPI use is well established in meta-analyses [], as the lack of gastrointestinal acid can significantly alter the microbiome towards Clostridia []. Our findings that suggest CDE risk is nearly tripled from PPI exposure strengthens the notion that acid blockade should be used judiciously in patients at risk for all types of CDI, including CDE. Secondly, having a colectomy for IBD seems to increase CDE risk 3-fold, similar to the increased risk for traditional CDI from IBD []. The strong concordance of CDE with established CDI risk factors suggests a host-pathogen–specific mechanism is responsible (eg, PPI increasing C. difficile proliferation and IBD increasing enteral inflammation) independent of colon-specific factors.
Regarding prior antibiotic use, our unadjusted analysis resulted in a similar increased risk associated with cephalosporin and β-lactam as those in large meta-analyses of CDI [, ]. However, in our population these associations became less significant when we included multiple variables, suggesting other factors may have a more significant effect on the microbiome in a postcolectomy population. For example, enteral nutrition was suggested to be protective, likely due to the nutrient-rich environment effect on maintaining the microbiome []. Surprisingly, if patients on enteral nutrition also received β-lactams, there appeared to be a synergistic effect towards the risk of developing CDE (HR, 17.83; 95% CI, 2.75–115.68; P = .003). Teasing apart a possible complex interaction between antibiotics and enteral feeds predisposing a patient to CDE is beyond the scope of this study, but the striking interaction could be considered in future mechanistic studies.
Our findings indicate that CDE often presents as severe, fulminant disease with over 80% of patients requiring inpatient hospitalization. A similar predilection for CDE to present as severe disease has been noticed in prior case reports and case series [, , ]. Possible reasons could include an immunologic difference between postcolectomy patients (many of which have IBD or are immunosuppressed at baseline), a later presentation, or a delay in diagnosis considering the rarity and prior disbelief in CDE. To avoid the latter, providers should keep CDE in their differential and send stool testing in a postcolectomy patient presenting with diarrhea and abdominal pain.
Insomuch as our research objective was to characterize an extremely rare disease and discover possible risk factors, our study has multiple limitations. We chose a retrospective cohort design due to the rarity of CDE, which introduces limitations and unmeasured confounding that may not be abrogated despite our careful analysis. To avoid misclassification bias of CDE cases, extensive chart review was performed by multiple study members to assure cases had total (rather than subtotal) colectomy and met our CDE definition; we included clinical symptomatology and sensitive PCR and toxin testing to reduce the chance of diagnosing colonization as CDE. Still, there may have been misclassification of CDE cases as controls due to inadequate testing during the early years of our cohort, when recognition of CDE as a clinical entity was much lower than subsequent years. Selection bias is also a possible limitation in that our surveillance was hospital-based, resulting in a high proportion of severe CDE cases; however, we believe this is insignificant as CDE evaluation or treatment is unlikely to occur as an outpatient. In addition to selection bias, our analysis extending over 20 years of data results in the collection of CDE cases from time periods with vast differences in C. difficile epidemiology, testing recommendations, antibiotic stewardship measures, treatment options, and overall quality of postcolectomy care. These potential confounders limit use of the data to the analysis presented, without the ability to evaluate secular trends or temporal differences in CDE. Despite this limitation, we believe our case collection over such an extensive time period is a unique strength of our study, as the numbers of cases needed for a discovery analysis would not be possible otherwise. In order to generate possible risk factors, antibiotic exposure had to be compared in the month prior to surgery, creating a limitation in that risk is assumed to be highest in the first 3 months after antibiotic exposure, as it is in CDI []. However, 20 of 44 (45.5%) of our cases were within 90 days of colectomy and nearly half (46%) of patients with late onset CDE were given antibiotics. Additionally, there is growing evidence that antibiotic exposure can have long-term (months to years) microbiome effects [, ]. A limitation of our analysis suggesting a possible synergistic interaction between β-lactams and enteral feeding towards CDE is our inability to confirm this finding or further explore a mechanism; it is plausible that there could be a contribution from β-lactams predisposing to colonization, then having diarrhea from enteral feeding. Finally, our multivariable analysis did not include many risk factors associated with CDE in the unadjusted analysis. It is still possible that these variables and/or additional unaccounted risk factors do contribute to CDE, but we lacked the statistical power to demonstrate it with the low overall number of CDE cases in our cohort.
Clostridioides difficile enteritis is a legitimate entity to include on the differential for postcolectomy patients presenting with fever, abdominal pain, and increased stool output. Clostridioides difficile enteritis occurs after 5.1% of colectomies and usually within 1 year but can be seen decades afterwards. The presentation of CDE is frequently severe, requiring hospitalization. Possible risk factors include colectomy for IBD or CDI and acid suppression. A complex interaction between β-lactams and enteral feeds may amplify CDE risk. Providers must be aware that the risk of C. difficile does not end with total colectomy, so they should be vigilant in identifying and treating CDE.
Acknowledgements
Disclosures : K. R. has consulted with Bio-K Plus International and is a principal investigator on an investigator-initiated grant from Merck & Company, Inc.
Financial support : This work was supported by grants from the Claude D. Pepper Older Americans Independence Center (grant number AG- 024824) and the Michigan Institute for Clinical and Health Research (grant number 2UL1TR000433). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest : All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bartlett JG, Moon N, Chang TW, et al Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology1978; 75:778–82.
- 2. Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis2012; 55:216–23.
- 3. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis2012; 55(Suppl 2):S65–70.
- 4. Magill SS, Edwards JR, Bamberg W, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med2014; 370:1198–208.
- 5. Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med1998; 49:375–90.
- 6.
- 7. Seril DN, Shen B. Clostridium difficile infection in the postcolectomy patient. Inflamm Bowel Dis2014; 20:2450–69.
- 8. LaMont JT, Trnka YM. Therapeutic implications of Clostridium difficile toxin during relapse of chronic inflammatory bowel disease. Lancet1980; 1:381–3.
- 9. Kim JH, Muder RR. Clostridium difficile enteritis: a review and pooled analysis of the cases. Anaerobe2011; 17:52–5.
- 10. Killeen S, Martin ST, Hyland J, O'Connell PR, Winter DC Clostridium difficile enteritis: a new role for an old foe. Surgeon2014; 12:256–62.
- 11. Hayetian FD, Read TE, Brozovich M, Garvin RP, Caushaj PF Ileal perforation secondary to Clostridium difficile enteritis: report of 2 cases. Arch Surg2006; 141:97–9.
- 12. Boland E, Thompson JS. Fulminant Clostridium difficile enteritis after proctocolectomy and ileal pouch-anal anastamosis. Gastroenterol Res Pract2008; 2008:985658.
- 13. Lavallée C, Laufer B, Pépin J, Mitchell A, Dubé S, Labbé AC. Fatal Clostridium difficile enteritis caused by the BI/NAP1/027 strain: a case series of ileal C. difficile infections. Clin Microbiol Infect2009; 15:1093–9.
- 14. Thai H, Guerron AD, Bencsath KP, Liu X, Loor M. Fulminant Clostridium difficile enteritis causing abdominal compartment syndrome. Surg Infect (Larchmt)2014; 15:821–5.
- 15. Gagandeep D, Ira S. Clostridium difficile enteritis 9 years after total proctocolectomy: a rare case report. Am J Gastroenterol2010; 105:962–3.
- 16. Papaconstantinou I, Zampeli E, Dellaportas D, et al Synchronous cytomegalovirus and Clostridium difficile infection of the pouch: a trigger for chronic pouchitis? Clin J Gastroenterol 2014; 7:132–5.
- 17. Drudy D, O'Donoghue DP, Baird A, Fenelon L, O'Farrelly C. Flow cytometric analysis of Clostridium difficile adherence to human intestinal epithelial cells. J Med Microbiol2001; 50:526–34.
- 18. Testore GP, Nardi F, Babudieri S, Giuliano M, Di Rosa R, Panichi G. Isolation of Clostridium difficile from human jejunum: identification of a reservoir for disease?J Clin Pathol1986; 39:861–2.
- 19. Apel R, Cohen Z, Andrews CW Jr, McLeod R, Steinhard H, Odze RD Prospective evaluation of early morphological changes in pelvic ileal pouches. Gastroenterol1994; 107:435–43.
- 20. Neut C, Bulois P, Desreumaux P, et al Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol2002; 97:939–46.
- 21. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology2011; 140:1785–94.
- 22. Eriksson C, Cao Y, Rundquist S, et al Changes in medical management and colectomy rates: a population-based cohort study on the epidemiology and natural history of ulcerative colitis in Örebro, Sweden, 1963–2010. Aliment Pharmacol Ther2017; 46(8):748–57.
- 23. Ross H, Steele SR, Varma M, et al; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum2014; 57:5–22.
- 24. Church JM, Ashburn JH. Regarding the Clinical Practice Guidelines for the Surgical Treatment of Patients With Lynch Syndrome. Dis Colon Rectum2017; 60:e595–6.
- 25. Hoste EA, Clermont G, Kersten A, et al RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care2006; 10:R73.
- 26. Levy MM, Fink MP, Marshall JC, et al 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med2003; 31:1250–6.
- 27. Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother2012; 67:742–8.
- 28.
- 29. Shen BO, Jiang ZD, Fazio VW, et al Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol2008; 6:782–8.
- 30. DiDiodato G, McArthur L. Evaluating the effectiveness of an antimicrobial stewardship program on reducing the incidence rate of healthcare-associated Clostridium difficile infection: a non-randomized, stepped wedge, single-site, observational study. PLOS ONE2016; 11:e0157671.
- 31. Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol2018; 53:84–94.
- 32. Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE Gastric microbiota is altered in oesophagitis and Barrett's oesophagus and further modified by proton pump inhibitors. Environ Microbiol2014; 16:2905–14.
- 33. Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol2007; 5:339–44.
- 34. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother2013; 57:2326–32.
- 35. Deshpande A, Pasupuleti V, Thota P, et al Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother2013; 68:1951–61.
- 36. Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol2017; 30:45–53.
- 37. Freiler JF, Durning SJ, Ender PT. Clostridium difficile small bowel enteritis occurring after total colectomy. Clin Infect Dis2001; 33:1429–31.
- 38. Holmer C, Zurbuchen U, Siegmund B, Reichelt U, Buhr HJ, Ritz JP. Clostridium difficile infection of the small bowel–two case reports with a literature survey. Int J Colorectal Dis2011; 26:245–51.
- 39. Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J2007; 1:56–66.
- 40. De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol2005; 43:5588–92.