Leptospirosis is considered to be a rare disease in industrialized countries [, ]. However, leptospires are existent worldwide [], and due to changing recreational behavior (eg, increasing popularity of adventure racing []) and ecological changes (eg, global warming, heavy rainfalls, and flooding), this disease may also be on the verge of emerging in industrialized countries []. We report a cluster of 3 cases with acquisition of leptospirosis at the same location during river surfing on a standing wave in Switzerland.
METHODS
Study Participants
Leptospirosis was diagnosed in Case 1 and Case 2 during routine clinical work. Based on detailed patient history of these cases, acquisition of leptospirosis at 1 surfing spot was suspected. Contact to Case 3 was enabled by the prior 2 cases. All described patients provided written informed consent before publication.
Leptospira-Specific Laboratory Analyses
All Leptospira-specific enzyme immunoassays (EIAs) were performed with the commercially available kits SERION ELISA classic Leptospira IgG and SERION ELISA classic Leptospira IgM (virion/serion, Würzburg, Germany) according to manufacturer's instructions. Microscopic agglutination tests (MATs) were done at the Bavarian State Office for Health and Food Safety following standard procedure []. Microscopic agglutination tests included the serovars (sv) Australis, Autumnalis, Ballum, Bataviae, Bratislava, Canicola, Copenhageni, Grippotyphosa, Hardjo, Hebdomadis, Javanica, Icterohaemorrhagiae, Pomona, Pyrogenes, Saxkoebing, Sejroe, and Tarassovi. Each month, Leptospira sv are checked by control sera provided by the Royal Tropical Institute of Amsterdam. Identical amounts of patient's serum and live antigen were mixed and incubated at 20 to 30°C and then assessed by dark field microscopy. An agglutination of at least 50% identified in dark-field microscopy was found to be positive. Titers of reactive sera were determined by stepwise dilution starting from 1:12.5. A positive threshold of 1:100 was used for all sv.
Case 1
A 33-year-old male without relevant prior medical history reported sudden onset of malaise, fever, myalgia, and headache. After persistence of these symptoms for 4 days, he turned to a general practitioner. A viral infection was suspected, and symptomatic treatment with a nonsteroidal anti-inflammatory drug (NSAID) was initiated. Due to worsening of symptoms, he visited an emergency room of a city hospital on the next day. At presentation, the patient appeared ill and physical examination was unremarkable (in particular, there were neither skin findings nor conjunctival suffusions). Laboratory results revealed slightly elevated transaminase levels (alanine aminotransferase 133 U/L [normal range: <50 U/L], aspartate aminotransferase 115 U/L [normal range: <50 U/L]), acute kidney injury (maximum creatinine 146 µmol/L [normal range: 62–106 µmol/L]), increased C-reactive protein (maximum 174 mg/L [normal range: <5.0 mg/L]), slight thrombocytopenia (101 G/L [normal range: 150–450 G/L]), and leukopenia (2.7 G/L [normal range: 3.6–10.5 G/L]) with lymphopenia (0.5 G/L [normal range: 1.1–4.5 G/L]). Urine chemistry was noticeable for proteinuria of approximately 500 mg/day. Because medical history was remarkable for recent meningitis of his spouse, and given the clinical presentation of headaches associated with slight photophobia, a lumbar puncture was performed after an unremarkable cranial computed tomography. Cerebrospinal fluid (CSF) examination showed a normal cell count and no signs of a functional disorder of the blood-brain barrier. With respect to nonpathological CSF analysis and absence of meningism in physical examination, the differential diagnosis meningitis seemed unlikely. The patient was hospitalized and symptomatic treatment was continued. Serologic testing for hepatitis viruses A, B, and C was negative, with exception of positive results for anti-hepatitis A immunoglobulin (Ig) G, which resulted from prior vaccination. There was no indication of a recent cytomegalovirus (CMV) or Epstein-Barr virus (EBV) infection (CMV IgM and CMV IgG: negative; CMV-polymerase chain reaction (PCR): negative; EBV IgM: negative; EBV IgG: positive; EBV-PCR: negative). Polymerase chain reaction of the CSF was negative for CMV, EBV, herpes simplex virus 1 and 2, and varicella-zoster virus (VZV). Blood cultures remained without growth. Abdominal ultrasound demonstrated discrete splenomegaly of 14 cm and no postrenal cause of renal failure.
Repeated human immunodeficiency virus (HIV) screening tests performed with Roche COBAS Combo-Screening were borderline positive. Symptomatic therapy and intravenous hydration resulted in clinical improvement. The patient left the hospital after 4 days. Because clinical findings and laboratory results were considered compatible with a primary HIV infection, the patient was referred to the outpatient clinic of the University Hospital Zurich.
For confirmatory reasons, HIV testing was redone using an Abott Architect Combo-Screening test, HIV-1 plasma RNA-PCR, testing for p24-Antigen, and a Western blot. All of these results turned out to be negative, and the diagnosis of primary HIV infection was discarded.
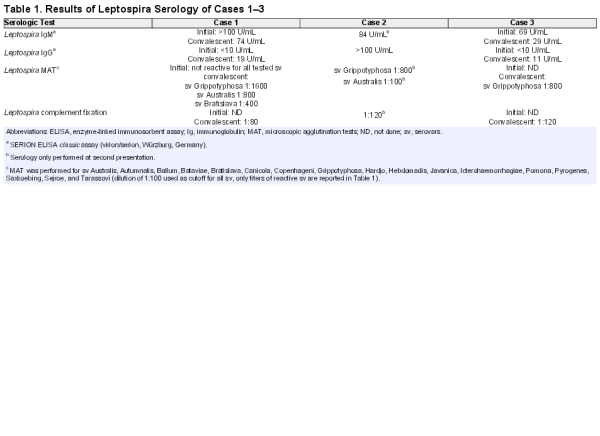
During the initial hospital stay, leptospirosis was considered and serologic testing was prompted. With a delay of a few days, Leptospira EIA reported IgM > 100 U/mL and undetectable IgG (SERION ELISA classic assays). Leptospira MAT remained negative. Because these results were inconclusive, testing was repeated after 5 weeks. Leptospira infection was confirmed with seroconversion (Leptospira IgM 74 U/mL, IgG 19 U/mL) and a MAT titer of 1:1600 for serovar Grippotyphosa, 1:800 for serovar Australis, and 1:400 for serovar Bratislava (Table 1). The patient reported full recovery without any sequelae.
After detailed anamnesis, occupational risk factors or acquisition abroad were excluded, but river surfing as recreational activity was revealed. During this activity, swallowing of water occasionally occurred. In particular, the patient reported frequent surfing, approximately 2 times per week, on a standing wave of a river in Switzerland (Reuss River in Aargau). He surfed the last time on this river 7 days before onset of symptoms. This surfing spot turned out to be the most probable infection source.
Case 2
A 32-year-old female—the spouse of Case 1—without any relevant previous medical history reported sudden onset of malaise and dizziness followed by fever, myalgia, and severe headache associated with photophobia and phonophobia (∼2 weeks before presentation of Case 1). As symptoms persisted, despite self-initiated therapy with acetaminophen and NSAIDs, she visited a general practitioner. A viral syndrome was suspected, and symptomatic therapy with hydration and analgesia was continued. Several consultations at the general practitioner ensued as symptoms deteriorated, despite successively extended analgesic therapy. The patient finally visited an emergency room as her headaches became intolerable (6 days after onset of symptoms). She was subfebrile and appeared ill and anguished; at clinical examination, meningism was present. Cranial computed tomography identified no intracranial bleeding, no sinus venous thrombosis, or mass lesions. Laboratory analysis revealed moderately increased C-reactive protein (19 mg/L [normal range: <5.0 mg/L]) and slightly elevated alkaline phosphatase (132 U/L [normal range: 40–130 U/L]). Transaminases, creatinine, and white blood count (WBC) were within the normal range. Lumbar puncture yielded an elevated cell count (196/µL [normal range: <6/µL]) with a predominance of neutrophils and elevated CSF proteins (0.86 g/L [normal range: 0.23–0.38 g/L). Empiric therapy with acyclovir and ceftriaxone was initiated. Immediately after application of the first dose of ceftriaxone, redness, swelling, and hyperthermia of the extremities occurred. Because her anamnesis included a penicillin allergy, an allergic reaction to ceftriaxone was suspected and steroids and antihistamines were administered once. A viral pathogenesis of the meningitis was favored, and antibiotic therapy was consequently discontinued. Because adenovirus-PCR, CMV-PCR, herpes simplex-PCR and VZV-PCR turned out to be negative, acyclovir was also stopped. Blood and liquor cultures showed no bacterial growth. After 1.5 weeks, the patient left the hospital still requiring analgesic combination therapy with metamizole and oxycodone for her headaches. After a period of clinical improvement lasting ∼1 week, she suffered from progressive malaise, relapsing headaches, and nausea, necessitating again admission to a hospital. Since by this time her partner was suspected of suffering from leptospirosis, she was also tested for Leptospira infection. Leptospira antibodies were detected by EIA (IgM 84 U/mL, IgG > 100 U/mL; SERION ELISA classic assays), and MAT confirmed infection by the serovar Grippotyphosa (titer 1:800 for serovar Grippotyphosa, 1:100 for serovar Australis). Due to the severity of symptoms, antibiotic therapy with doxycycline was administered. She experienced progressive improvement within a few days after initiation of therapy, but overall recovery was slow.
Similar to Case 1, this patient reported surfing on the same river twice weekly. She remembered the last exposure to river water 2 days before symptom onset. No occupational exposure or contacts with pets existed, and her last stay abroad was more than 1.5 months before illness. In consideration of the maximum incubation period of 30 days, acquisition of leptospirosis most likely occurred in the identical river where her spouse contracted the infection.
We discussed with Cases 1 and 2 our suspicion that surfing on this certain river was the source of infection. Subsequently, they asked their peer group of river surfers whether they had any symptoms suggestive of leptospirosis and were able to identify at least 1 more affected person (ie, Case 3).
Case 3
Case 3 is a 34-year-old male who belongs to the peer group of the previously described couple. He reported the onset of illness approximately 1 month before Case 2. He also had no relevant medical history. Symptoms started with malaise, arthralgia, fever, and chills. High fever persisted despite regular intake of acetaminophen. Due to illness, he remained in bed most of the time. After 4 days of illness, he sought medical attention at an emergency room. At presentation, he appeared in a slightly reduced general condition, and his vital parameters were within normal range with exception of a body temperature of 37.9°C. His physical examination was unremarkable. Laboratory results revealed a slightly elevated creatinine (110 µmol/L [normal range: 62–106 µmol/L]), mild hyponatremia (133 mmol/L [normal range: 135–145 mmol/L]), elevated total bilirubin (61 µmol/L [normal range: <21 µmol/L]), and increased C-reactive protein (74 mg/L [normal range: <5.0 mg/L]). His blood count was remarkable for mild thrombocytopenia (97 G/L [normal range: 150–450 G/L]) and left shift (total WBC within the normal range). Because the patient reported holidays in Indonesia 3 months earlier, malaria was ruled out after repeated testing. Blood cultures were negative. Serologic testing for HIV, viral hepatitis, schistosomiasis, amebas, and filariasis showed no signs of infection. Again, a viral syndrome was suspected and symptomatic treatment continued. After 1 week, symptoms started to wane. Subsequently, the patient suffered from severe headaches associated with phonophobia and photophobia, fever reappeared, and scrotal pain with swelling was present. This time, symptoms persisted for 1 week and ended with a gradual weakening of illness. The patient arranged for an appointment with an Infectious Diseases specialist because he mistrusted the diagnosis of a viral infection. At consultation, physical examination was unremarkable, but the patient reported almost full recovery at that time. Differential blood count, creatinine, transaminases, bilirubin, and C-reactive protein were within the normal range. Serologic testing revealed elevated Leptospira IgM (69 U/mL) with absence of Leptospira IgG (SERION ELISA classic assays). Leptospirosis was favored, and because the patient felt healthy again, no treatment was initiated. Overall, symptoms lasted for approximately 1 month. Convalescent sera testing verified leptospirosis caused by serovar Grippotyphosa (IgM 29 U/mL, IgG 11 U/mL, MAT 1:800 for serovar Grippotyphosa).
Also in this case, no occupational exposure was present. This patient had been surfing on the suspect river in high frequency (approximately 4 times per week) for approximately 6 weeks before onset of illness. The last exposure to river water was reported 3 days before appearance of initial symptoms.
DISCUSSION
To our knowledge, this case series is the first cluster of leptospirosis associated with surfing on a river in Switzerland. In 2012, a single case of leptospirosis was traced back to the same river and even to the identical surfing spot []. This 28-year-old male presented with sudden onset of fever, chills, headaches, myalgia, and arthralgia; laboratory examinations were remarkable for signs of inflammation, acute renal failure, and elevated transaminases. In consideration of a bacterial infection, this patient received antibiotic therapy with cephalosporines, resulting in rapid clinical and laboratory improvement. In this case, MAT identified an infection with Leptospira serovar Bratislava. It seems likely that more undiagnosed persons acquired leptospirosis through surfing on this river. A 4th case of leptospirosis was suspected, because another surfer mentioned that he had also suffered from a prolonged period of compatible clinical findings. However, this person refused to undergo a detailed interview and serological testing at our outpatient clinic.
Leptospirosis is a worldwide zoonosis. In addition, leptospirosis is still often considered to be a disease of tropical and subtropical regions and to occur generally in resource-limited settings, but leptospirosis also occurs in resource-rich settings. Switzerland lacks an adequate surveillance system for this disease because human cases are not subjected to mandatory report for more than 15 years. Data from Europe indicated that the highest rates of leptospirosis occur in summer and fall [, ]. Likewise, all described patients in this study suffered from leptospirosis either in summer (Case 2 in September 2014, Case 3 in August 2014) or in fall (Case 1 in October 2014). This seasonal pattern may reflect the fact that survival of leptospires depends on warm and humid conditions or that the risk of acquisition grows with increasing human water activities during hot summer days. Indeed, mean water temperature, recorded at an observation station approximately 6 miles downstream (Mellingen, Switzerland), was higher in these months (18.4°C in August 2014, 17.8°C in September 2014, 15.3°C in October 2014) than the annual average (12.7°C) [].
Infection of humans occurs via skin lesions, mucous membranes, and less frequently via inhalation or ingestion. In our case series, infection may have resulted from swallowing water or macerated or abraded skin during surfing [, ], but none of the reported cases remembered having had skin lesions. Several risk groups due to occupational exposure have been identified for leptospirosis, including, for example, farmers, veterinarians, butchers, and pet sellers, but there is also increasing evidence for recreational exposure [, , ]. For physicians, the association between recreational activity and leptospirosis may not be well known, which can result in significant underdiagnosis of leptospirosis. With changes in recreational activities, such as the growing popularity of extreme sports and adventure racing, an increase in risk for acquisition of leptospirosis is imaginable. This hypothesis is also supported by data from the Netherlands that indicated a high proportion of acquisition of leptospirosis during recreational activities for both autochthonous and imported infections []. The incubation period typically lasts 5–14 days. Given the high frequency of river surfing for all reported cases (2–4 times/week), we were not able to identify the most probable date of acquisition. The severity of the disease encompasses asymptomatic disease to fatal fulminant infection []. The clinical course is often biphasic and characterized by an acute phase with fever, headache, myalgia, abdominal pain, and an immunogenic phase, which may be associated with severe disease manifestations. Initially, all cases suffered from unspecific malaise, myalgia or arthralgia, and fever, which was misinterpreted as viral syndrome. Two of the 3 cases displayed reworsening of symptoms after a short period of improvement, only Case 1 described a continuous deterioration. Meningeal involvement presenting as aseptic meningitis (shown in Case 2) has recently been reported at a frequency of 5%, and older data have documented even higher rates [, ]. Consistent with literature, the 2 cases with bimodal presentation experienced reappearance of subfebrile temperatures or fever during the immunogenic phase. Severe manifestations encompass Weil's disease (characterized by icterus, acute renal failure, bleeding disorders), renal failure, leptospirosis-associated pulmonary hemorrhage syndrome, and, rarely, myocarditis []. Diagnosis is complicated by protean symptoms and several difficulties of diagnosis. Despite several limitations, MAT is considered as reference serologic test method []. The MAT is limited to few expert laboratories because this test requires living leptospires. A MAT performed in the early course of leptospirosis can deliver negative results and may take up to 3–4 weeks to become reactive [, ]. This is compatible with the initial negative MAT in Case 1, which developed reactivity in the convalescent sample. Positive MAT results to multiple sv are likely due to frequent cross-reactions between sv [, ] (Table 1). Nevertheless, the highest titers were recorded for serovar Grippotyphosa in all 3 cases, thus indicating that all patients were infected by the same serovar and likely at the same infection source. Leptospira-specific EIAs are approved for diagnostics, but positive results need to be confirmed by MAT. Culture requires specialized culture media, and it is insensitive and time-consuming. In addition, culture might remain without growth due to unspecific media used at first choice; also, in this case series, unspecific culture media (containing tryptic soy broth) were applied. Clinical and laboratory criteria recommended by the World Health Organization for the diagnosis of leptospirosis are summarized in Figure 1 []. Diagnosis of leptospirosis requires explicit clinical suspicion of this disease to prompt sufficient testing.

Figure 1
Clinical description and recommended case definition according to the World Health Organization (WHO). Abbreviations: ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; PCR, polymerase chain reaction.
The repeated false-positive HIV screening test performed with Roche Combo-screening of Case 1 seems remarkable. Several conditions have been associated with false-positive results such as pregnancy, autoantibodies, hepatitis, cancer, intravenous drug use, and influenza or rubella vaccination []. Weber et al [] reported false-positive results using Roche COBAS fourth-generation HIV screening tests at a rate of 0.41%. False-positive results were found at the highest frequency in patients with detectable anti-hepatitis A IgM, pregnant women, and patients suffering from leukemia []. To avoid the impact of a false-positive result, repeated testing using different types of tests and repeated samples for diagnosis of HIV is mandatory []. Our findings suggest that leptospirosis might also cause false-positive HIV test results.
In case of severe leptospirosis, most physicians will prefer antibiotic treatment because some data showed a decreased duration of illness with antibiotic therapy []. In a Cochrane review, no significant benefit of antibiotic treatment was shown []. Case 2 reported a redness, hyperthermia, and swelling of the distal extremities after application of ceftriaxone. Because her medical history was positive for a cutaneous reaction after use of penicillin, an allergic reaction seemed likely. Alternatively, a Jarisch Herxheimer reaction might have been causative []. Jarisch Herxheimer reactions are described for all spirochetal infections. Administration of antibiotics causes elimination of spirochetes associated with an acute inflammatory response and release of large amounts of cytokines. Because these symptoms occurred in the early course of the disease, ongoing leptospiremia seems possible, which fits for the differential diagnosis Jarisch Herxheimer reaction.
CONCLUSIONS
Most relevant reservoirs of leptospires worldwide are rodents. Leptospires are excreted via urine [, ] and can survive for weeks or months in moist soil or water. In this case series, the reservoir of leptospires might be rodents living along the river. It is interesting to note that leptospirosis was recently identified as cause of death in free-ranging Eurasian beavers (Castor fiber) from the related Aare river [], thus confirming the presence of leptospires in the environment and corroborating the river as probable infectious source. However, although beavers are rodents, they might be only incidental hosts, because they develop fatal illness. Data on small rodents in Switzerland are scarce. Only 1 study showed a renal carriage of leptospires at a rate of 12.6% in small mammals from the city of Zurich []. Detailed research with serologic testing and PCR of kidney tissue from beavers, small rodents, and other wildlife species from various Swiss regions is in progress, to obtain a better picture of the geographical distribution and prevalence of different Leptospira sv in potential animal reservoirs. Furthermore, an emergence of leptospirosis has been observed in domestic dogs, with a majority of recorded cases originating from the Swiss Plateau, including the region of the human and beaver cases []. Canine leptospirosis exhibits a high similarity to human disease, for example, renal, pulmonary, hemorrhagic, and liver manifestations are common, and numerous identical sv can cause disease in both humans and dogs. Therefore, dogs may be considered to be a model. The emergence of this disease in animals should trigger a raising awareness of human disease. At the University of Zurich (Professor Dr. Hatz, Epidemiology, Biostatistics and Prevention Institute), a current research project aims at assessing the relevance of human leptospirosis in Switzerland. Swiss hospitals have been contacted to report cases of human leptospirosis diagnosed in the last years and the assumed mode of acquisition. Based on these data, an estimate of human leptospirosis cases will be gathered that should help to evaluate trends in human disease. If these data indicated a parallel increase in human leptospirosis, efforts to change the status of leptospirosis from a nonnotifiable to a notifiable disease would be reinforced. Our work strongly supports a cross-species “One Health” approach with further collaborations between human and veterinary medicine.
Acknowledgments
We thank the 3 patients for their willingness to participate in this study.
Financial support. This study was supported by the “Clinical Research Priority Program: Viral Infectious Diseases” of the University of Zurich (to H. F. G. and P. W. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis2005; 18:376–86.
- 2. Pappas G, Papadimitriou P, Siozopoulou V, et al. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis2008; 12:351–7.
- 3. World Health Organization. Human leptospirosis: guidance for diagnosis, surveillance and control. 2003.
- 4. Stern EJ, Galloway R, Shadomy SV, et al. Outbreak of leptospirosis among Adventure Race participants in Florida, 2005. Clin Infect Dis2010; 50:843–9.
- 5. Morgan J, Bornstein SL, Karpati AM, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis2002; 34:1593–9.
- 6. Haake DA, Dundoo M, Cader R, et al. Leptospirosis, water sports, and chemoprophylaxis. Clin Infect Dis2002; 34:e40–3.
- 7. Sejvar J, Bancroft E, Winthrop K, et al. Leptospirosis in “Eco-Challenge” athletes, Malaysian Borneo, 2000. Emerg Infect Dis2003; 9:702–7.
- 8. Sanders EJ, Rigau-Perez JG, Smits HL, et al. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996 [correction of 1966]. Am J Trop Med Hyg1999; 61:399–404.
- 9. Trevejo RT, Rigau-Perez JG, Ashford DA, et al. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J Infect Dis1998; 178:1457–63.
- 10. Jevon TR, Knudson MP, Smith PA, et al. A point-source epidemic of leptospirosis. Description of cases, cause, and prevention. Postgrad Med1986; 80:121–2, 7–9.
- 11. Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect2011; 17:494–501.
- 12. Staudenmann D, Rossi M. [Kopfschmerzen vom Surfen]. Schweiz Med Forum2013; 13:212–3.
- 13.
- 14. van Alphen LB, Lemcke Kunoe A, Ceper T, et al. Trends in human leptospirosis in Denmark, 1980 to 2012. Euro Surveill2015; 20:33–41.
- 15.
- 16. Corwin A, Ryan A, Bloys W, et al. A waterborne outbreak of leptospirosis among United States military personnel in Okinawa, Japan. Int J Epidemiol1990; 19:743–8.
- 17. Tunbridge AJ, Dockrell DH, Channer KS, McKendrick MW. A breathless triathlete. Lancet2002; 359:130.
- 18. Abb J. Acute leptospirosis in a triathlete. Wilderness Environ Med2002; 13:45–7.
- 19. Goris MG, Boer KR, Duarte TA, et al. Human leptospirosis trends, the Netherlands, 1925–2008. Emerg Infect Dis2013; 19:371–8.
- 20. Ashford DA, Kaiser RM, Spiegel RA, et al. Asymptomatic infection and risk factors for leptospirosis in Nicaragua. Am J Trop Med Hyg2000; 63:249–54.
- 21. Levett PN. Leptospirosis. Clin Microbiol Rev2001; 14:296–326.
- 22. Mendoza MT, Roxas EA, Ginete JK, et al. Clinical profile of patients diagnosed with leptospirosis after a typhoon: a multicenter study. Southeast Asian J Trop Med Public Health2013; 44:1021–35.
- 23. Voon V, Saiva L, Prendiville B, et al. Leptospiral myocarditis--a rare assault on myocardium. Int J Cardiol2014; 172:e76–8.
- 24. Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis2003; 3:757–71.
- 25. Navinan MR, Rajapakse S. Cardiac involvement in leptospirosis. Trans R Soc Trop Med Hyg2012; 106:515–20.
- 26. Cumberland P, Everard CO, Levett PN. Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am J Trop Med Hyg1999; 61:731–4.
- 27. Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2nd ed. Melbourne: MediSci.1999.
- 28.
- 29. Araujo PR, Albertoni G, Arnoni C, et al. Rubella vaccination and transitory false-positive test results for human immunodeficiency virus Type 1 in blood donors. Transfusion2009; 49:2516–7.
- 30. Eguchi S, Takatsuki M, Soyama A, et al. False positivity for the human immunodeficiency virus antibody after influenza vaccination in a living donor for liver transplantation. Liver Transpl2013; 19:666.
- 31. Erickson CP, McNiff T, Klausner JD. Influenza vaccination and false positive HIV results. N Engl J Med2006; 354:1422–3.
- 32. Weber B, Gurtler L, Thorstensson R, et al. Multicenter evaluation of a new automated fourth-generation human immunodeficiency virus screening assay with a sensitive antigen detection module and high specificity. J Clin Microbiol2002; 40:1938–46.
- 33. (BAG) BfG. [Das schweizerische HIV Testkonzept – eine aktualisierte Übersicht]. 2013.
- 34. Watt G, Padre LP, Tuazon ML, et al. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet1988; 1:433–5.
- 35. Brett-Major DM, Coldren R. Antibiotics for leptospirosis. Cochrane Database Syst Rev2012; 2:CD008264.
- 36. Guerrier G, D'Ortenzio E. The Jarisch-Herxheimer reaction in leptospirosis: a systematic review. PloS One2013; 8:e59266.
- 37. Thiermann AB. The Norway rat as a selective chronic carrier of Leptospira icterohaemorrhagiae. J Wildl Dis1981; 17:39–43.
- 38. Giovannini SR, Ryser MP, Tagliabue S, et al. eds. Leptospirosis in European Beavers (castor fiber) from Switzerland. In: WDA/EWDA Conference. Lyon, France, 23-27 July2012.
- 39. Adler H, Vonstein S, Deplazes P, et al. Prevalence of Leptospira spp. in various species of small mammals caught in an inner-city area in Switzerland. Epidemiol Infect2002; 128:107–9.
- 40. Major A, Schweighauser A, Francey T. Increasing incidence of canine leptospirosis in Switzerland. Int J Environ Res Public Health2014; 11:7242–60.