Introduction
According to the Japanese Gastric Cancer Association (JGCA), National Cancer Comprehensive Network (NCCN) and European Society of Medical Oncology (ESMO), gastrectomy with D2 lymphadenectomy was considered as the standard treatment or was strongly recommended for advanced GC [-]. In China, there was a high incidence of stage III GC, and the prognosis of these patients was still poor. The obvious heterogeneity in survival of patients within stage III GC after D2 lymphadenectomy has not been well studied. Although the clinical prognostic value of the 8th edition staging system which was released by the American Joint Committee on Cancer (AJCC) in early 2016 was superior to the 7th edition, its guiding significance in clinical practice was still insufficient. Therefore, the Japanese JGCA system considered the location of the lymph node (LN) metastasis relative to the primary tumor as N stage. Besides, many studies [-] even reported that staging based on the metastatic lymph node ratio (MLR) or log odds of positive lymph nodes (LODDS) had more advantages than the TNM staging system. A standardized and globally accepted prediction system had yet to be widely adopted. Under this background, our department also established an ImmunoScore to predict recurrence and survival of GC in 2018 []. Present staging systems had limited ability to predict the prognosis of GC patients.
This study aimed to find some predictors of poor prognosis of patients with stage III GC based on the current staging system after distal or total gastrectomy with D2 lymphadenectomy. We intended to achieve some conclusions from a surgical aspect and expected that the results could complement the prognostic value of the present staging system.
Patients and Methods
Patients
A total of 320 stage III GC patients’ medical records who had undergone distal or total gastrectomy with D2 lymphadenectomy at the Department of General Surgery of Nanfang Hospital (Guangzhou, China) between September 2012 and January 2016 were retrospectively reviewed from a prospectively collected database [], written informed consents were obtained from all patients prior to entering their information into the database. The CLASS-01 studies [, ] were also conducted mainly based on this database. Some patients were restaged to a new classification according to the 8th edition of the TNM staging system. Patients with synchronous malignancies, proximal gastrectomy, neoadjuvant chemotherapy, no-radical operation and lost to follow-up in 3 years were excluded. Written informed consents were obtained from all patients prior to entering their information into the database. Adjuvant chemotherapy were platinum- and fluorouracil-based chemotherapy. The last follow-up was performed in January 2019.
Grouping Criteria
These patients were divided into 2 groups, the patients’ DFS in group 1 were ≥3 years, the patients’ DFS in group 2 were <3 years. The DFS in this study was defined as the time from operation to recurrence, death or to the last follow-up date. Most of the data came from the CLASS-01 studies [, ], and a 3-year of DFS was its cutoff point, so we also used a 3-year of DFS as cutoff point. Group 1 included 169 cases and group 2 included 151 cases. We compared clinicopathological features between these 2 groups by univariate analysis. In order to achieve some conclusions from a surgical aspect, we grouped the patients by distal and total gastrectomy, rather than by tumor locations. As the distal and total gastrectomy were mainstream surgical procedures, and the proximal subtotal gastrectomy had been abandoned by many clinics, we excluded proximal subtotal gastrectomy in this study. Then, we divided group 1 into group 1D and group 1T, and divided group 2 into group 2D and group 2T, according to distal or total gastrectomy. Group 1D included 101 cases who underwent distal gastrectomy; group 2D included 74 cases who underwent distal gastrectomy; group 1T included 68 cases who underwent total gastrectomy; group 2T included 77 cases who underwent total gastrectomy. Finally, we compared group 1D with group 2D, and group 1T with group 2T, respectively. The flow diagram is shown in Figure 1 (or online suppl. appendix, see http://www.karger.com/doi/10.1159/000512934).
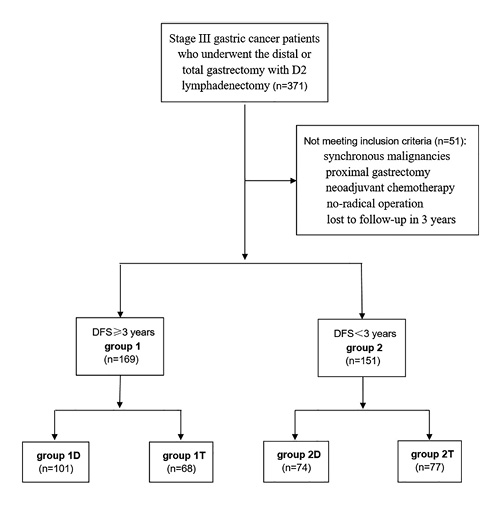
Fig. 1
Flow diagram.
The dissection of the perigastric LN of distal gastrectomy with D2 dissection included No. 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p and 12a; and that of total gastrectomy included No.1, 2, 3, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 10, 11p, 11d and 12a. We compared the status of LN metastasis in each group of perigastric LN between group 1D and group 2D, group 1T and group 2T, respectively. In our department, our surgeon performed the postoperative perigastric LN retrieval since 2012 (Fig. 2). Jiang et al. [] reported that the surgeon could do better than pathologists in the aspect of LN retrieval. The average number of LN retrieval in our department was 62.
Fig. 2
Our department’s surgeon performed the postoperative perigastric LN retrieval.
Statistical Analysis
χ2 test and Fisher’s exact test were used for univariate analysis; logistics regression analysis was used for multivariate analysis. The Cox proportional regression model was used for the analysis of DFS. The log-rank test was used for drawing the survival curves. All statistical analyses were performed using SPSS program (Statistical Product and Service Solution 20 for Windows; SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered as the limit for statistical significance.
Results
Clinical and Pathologic Characteristics
The patients’ detailed clinicopathological characteristics of groups 1 and 2 are presented in Table 1. Univariate analyses revealed that the patients’ LN metastasis was the only significant difference between groups 1 and 2 (p < 0.05; Table 1).
Patients’ Metastasis Status of Each LN Group
Then, we compared group 1D with group 2D, and group 1T with group 2T, respectively. Compared with group 1D, the percentage of patients who had metastatic LN in all groups of the perigastric LN (included No.1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p and 12a) increased in group 2D. Among these, the increases of group No.12a, 11p, 6 and 5 LN were significant (p < 0.05), especially the group No.12a LN (Table 2). Similarly, compared with group 1T, the situation in group 2T (included No.1, 2, 3, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 10, 11p, 11d and 12a) was the same. Among these, the increases of group No.12a, 11d, 8a, 6, 2 and 1 LN were significant (p < 0.05), especially the group No.12a LN (Table 2).
At distal gastrectomy, we chose No.5, No.6, No.11p and No.12a to conduct multivariate analysis. Besides, to avoid deviation, we chose the depth of invasion and adjuvant chemotherapy to conduct multivariate analysis (a value of p < 0.50 was set as the limit for multivariate analysis in Table 1). Multivariate logistics regression analyses revealed that only No.5 and No.12a showed significant difference (p < 0.05) between group1D and group 2D (Table 3).
At total gastrectomy, we chose No.1, No.2, No.6, No.8a, No.11d, No.12a, the depth of invasion and adjuvant chemotherapy to conduct multivariate analysis. Multivariate logistics regression analyses revealed that only No.6, No.8a and No.12a showed significant difference (p < 0.05) between group 1T and group 2T (Table 4).
When we combined distal and total gastrectomy, we found the metastasis of No.12a LN significantly increased in both distal and total gastrectomy, furthermore, its increase was the most significant (p < 0.01).
Among all our 320 patients, there were 314 patients with metastatic LN. Lastly, to investigate the effect of metastatic No.12a on the prognosis of patients, we divided the 314 patients into group 12a – and group 12a +. Group 12a – included 251 cases whose No.12a LN were nonmetastatic; group 12a + included 63 cases whose No.12a LN were metastatic. Survival of patients between group 12a – and group 12a + was compared.
The 3-year DFS rates of the group 12a – and group 12a + were 57.8 and 28.6%. The DFS rate of the group 12a – was significantly higher than that of the group 12a + (HR 2.088, 95% CI 1.495–2.915, p = 0.000). The survival curves of group 12a – and group 12a + for DFS are shown in Figure 3 (p = 0.000).
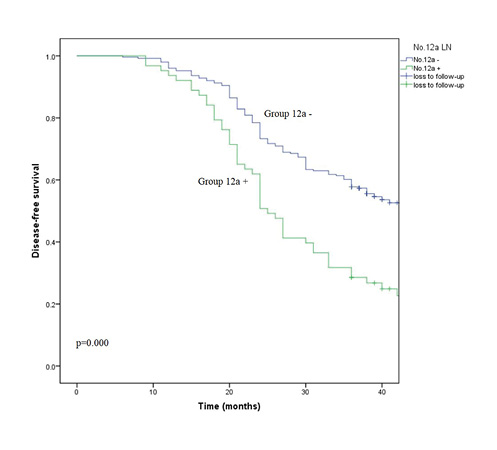
Fig. 3
Survival curves of group 12a – and group 12a + for DFS.
Among all our 320 patients, there were 180 patients that had metastatic No. 5, 6 and 12a LN (the right LNs). To clarify whether adjuvant chemotherapy affects survival of patients with metastatic right LNs, a survival analysis was conducted in these patients. The DFS rate of the patients with adjuvant chemotherapy (112 cases) was higher than that of the patients without adjuvant chemotherapy (68 cases), but the difference was not statistically significant (HR 1.29, 95% CI 0.89–1.89, p = 0.182). The survival curves of patients with adjuvant chemotherapy and patients without adjuvant chemotherapy for DFS are shown in Figure 4 (p = 0.182).
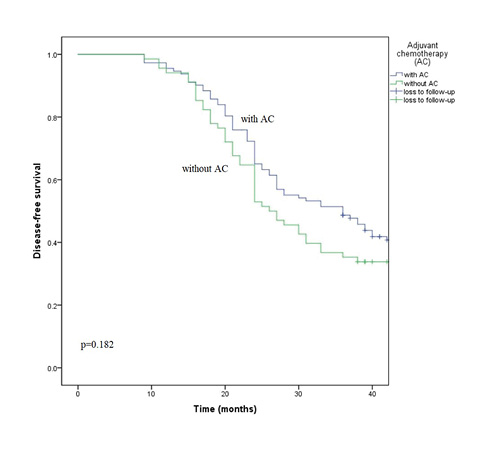
Fig. 4
Survival curves of patients with adjuvant chemotherapy and patients without adjuvant chemotherapy for DFS.
Discussion
Lymphatic metastasis is one of the most important poor prognostic factors in GC, LN-negative GC patients had a better prognosis than LN-positive patients [, ]. Woo et al. [] also developed a universally applicable prediction model to assess prognosis in GC patients, they thought it could supplant the TNM staging system. A standardized and globally accepted prediction system of GC has yet to be adopted. Even the clinicopathological prognostic factors of GC patients had also been controversial. For example, the prognostic implication of signet ring cell type gastric cancer still had debate []. Run-Cong et al. [] thought that the early signet ring cell type gastric cancer was associated with better prognoses, while the advanced one was associated with worse prognoses. GC in the young was an advanced disease with poor prognostic features []. The histological mixed-type of GC was a prognostic indicator in stage I GC []. Choi et al. [] proposed that the Lauren classification needed to be modified, they thought it should include the anatomical location of the tumor. Our study suggested that gender, age, tumor size, Lauren type, differentiation status, lymphovascular or nerve invasion and even depth of invasion might not be important prognostic factors in patients with stage III GC, but LN metastasis was an important prognostic factor in these patients. This was a valuable conclusion for the N staging system.
In general, differentiation status, lymphovascular or nerve invasion, depth of invasion, and tumor diameter are independent prognostic factors. However, in this study, we even found that the ratios of low/un-differentiation and lymphovascular or nerve invasion in group 1 were greater than those in group 2 (65.1 vs. 62.9%, 88.8 vs. 86.8%, respectively), this was just a univariate analysis, limitation was inevitable.
Our study indicated that the DFS rate of stage III GC patients with adjuvant chemotherapy was higher than that of stage III GC patients without adjuvant chemotherapy, but the difference was not statistically significant. It seemed that adjuvant chemotherapy was not an important reason which was associated with the prognosis of patients with stage III GC. However, as we know, chemotherapy has an important role in the treatment of advanced GC. Recently, many studies have focused on chemotherapy in GC. Kim et al. [] thought adjuvant XELOX chemotherapy was more effective than S-1 for patients with stage III B or III C GC after D2 dissection. Al-Batran et al. [] reported that the perioperative FLOT (fluorouracil plus leucovorin, oxaliplatin, and docetaxel) improved overall survival compared with perioperative ECF/ECX (epirubicin, cisplatin, and fluorouracil/capecitabine) in locally advanced, resectable gastric or gastro-esophageal junction adenocarcinoma. The difference could possibly be statistically significant if the sample size was large enough in our study. Further research in this area also would be needed.
Our study reconfirmed the importance of metastatic LN in the aspect of prognosis in stage III GC. We thought that the status of metastatic LN in the prognostic value of GC needed to be further enhanced in present staging systems. There was still debate about the minimum number of examined LNs for an accurate staging in GC patients. Biffi et al. [] suggested that the number of LN resection should be more than 15, it could significantly increase the DFS and overall survival. Lu et al. [] thought that a harvest of at least 21 LNs in radical total gastrectomy could yield a better prognosis. Kim et al. [] observed that pN3 GC patients should be considered to have a stage IV disease. We found that metastasis of No.12a and 5 LN in distal gastrectomy and metastasis of No.12a, 8a and 6 LN in total gastrectomy were the most significant poor prognostic indicators of stage III GC patients, respectively. As we know, No.12a, 8a and 5 LN were very near to each other in the anatomical position. To some extent, this could explain our conclusion. Feng et al. [] reported that GC patients with No. 5 or No.12a LN metastases may be accompanied with No. 12p and No. 12b LN metastases. It seemed that their conclusion was partly consistent with our results in distal gastrectomy. Through evaluation of the prognostic value of No.8p LN in GC patients, Guo et al. [] found that the positive No.8p LN was a poor prognostic factor for GC patients and should be recognized as a distant metastasis. Furthermore, among these metastatic LNs, we found that the metastasis of No.12a LN maybe was the most significant poor prognostic factor. We know that No.12a LN is the LN in the hepatoduodenal ligament along the hepatic artery. It was usually considered that patients with metastasis of No.12a LN would have more metastatic LNs, suggesting higher tumor burden. Our research further confirmed this situation. By studying the differences between 12aD+ group (with No.12a dissection) and 12aD–group (without No.12a dissection), Yang et al. [] reported that No.12a LN metastasis should not be considered as distant metastasis, they thought that No.12a lymphadenectomy was an independently better prognostic factor for stage III patients. This suggests that the prognostic value of No.12a LN deserves further study.
Our study has several limitations, first, in order to ensure the accuracy of data, we only reviewed the data from 2012 (because our surgeon performed the postoperative perigastric LN retrieval from this time, and most of the data came from the CLASS-01 study [, ]), so we did not investigate the long-term survival of our patients; second, many patients with T4 gastric cancer were included to this study, but we did not investigate the washing cytology; third, this was a retrospective study, bias was inevitable. Besides, the proximal subtotal gastrectomy had been abandoned by many clinics due to the high incidence of operative complication [], so we excluded proximal subtotal gastrectomy in this study.
In conclusion, poor prognosis of patients with stage III GC after D2 dissection is almost entirely due to lymphatic metastasis, the more metastatic LNs, the worse the patient’s prognosis. Although the 8th edition TNM staging system has further emphasized the importance of LN metastasis compare with the 7th edition, we still think that the status of LN metastasis in prognostic value of GC needs to be further enhanced, and we should pay more attention to the prognostic value of LN metastasis. Maybe the metastasis of No.12a LN is the most significant poor prognostic factor of patients with stage III GC after D2 dissection. However, in China, dissection of No.12a LN was not carefully performed for surgical safety reasons in some small-scale hospitals. Recent study pointed out the importance of dissection of No.12a LN in radical gastrectomy. These also were valuable conclusions for the N staging system.
Acknowledgements
The authors acknowledge the help of Kun Wang in English language editing.
Statement of Ethics
Patients’ medical records were retrospectively reviewed from a prospectively collected database at the Department of General Surgery of Nanfang Hospital (Guangzhou, China). Written informed consents were obtained from all patients prior to entering their information into the database. The CLASS-01 study was also conducted mainly based on this database. The study was approved by the Ethics Committee of Nanfang Hospital of Southern Medical University (approval No. NFEC2018–243).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
X.H. contributed to the conception; X.H. and H.L. contributed to the design; X.H. and Y.H. did the acquisition of data; X.H. and H.L. did the quality control of data and algorithms; X.H. did the data analysis and interpretation; X.H. and L.H. did the statistical analysis; X.H. and X.L. prepared the manuscript; X.H. and L.H. edited the manuscript; J.Y. and G.L. did the revision of the article.
References
- 1. Association JGJapanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
- 2. Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312.
- 3. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold DESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(5suppl 5):v38–49.
- 4. Zhang BY, Yuan J, Cui ZS, Li ZW, Li XH, Lu YY. Evaluation of the prognostic value of the metastatic lymph node ratio for gastric cancer. Am J Surg. 2014;207(4):555–65.
- 5. Calero A, Escrig-Sos J, Mingol F, Arroyo A, Martinez-Ramos D, de Juan M, et al Usefulness of the log odds of positive lymph nodes to predict and discriminate prognosis in gastric carcinomas. J Gastrointest Surg. 2015;19(5):813–20.
- 6. Komatsu S, Ichikawa D, Miyamae M, Kosuga T, Okamoto K, Arita T, et al Positive Lymph Node Ratio as an Indicator of Prognosis and Local Tumor Clearance in N3 Gastric Cancer. J Gastrointest Surg. 2016;20(9):1565–71.
- 7. Kim Y, Park SH, Kim KM, Choi MG, Lee JH, Sohn TS, et al The Influence of Metastatic Lymph Node Ratio on the Treatment Outcomes in the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) Trial: A Phase III Trial. J Gastric Cancer. 2016;16(2):105–10.
- 8. Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann Surg. 2018;267(3):504–13.
- 9. Hu YF, Yu J, Zhang C, Wang YN, Cheng X, Huang F, et al [Development and implementation of a clinical data mining system for gastric cancer surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13(7):510–5.
- 10. Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34(12):1350–7.
- 11. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et alChinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321(20):1983–92.
- 12. Jiang L, Yao Z, Zhang Y, Hu J, Zhao D, Zhai H, et al Comparison of lymph node number and prognosis in gastric cancer patients with perigastric lymph nodes retrieved by surgeons and pathologists. Chin J Cancer Res. 2016;28(5):511–8.
- 13. Adachi Y, Kamakura T, Mori M, Baba H, Maehara Y, Sugimachi K. Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg. 1994;81(3):414–6.
- 14. Wu ZY, Li JH, Zhan WH, He YL, Wan J. Effect of lymph node micrometastases on prognosis of gastric carcinoma. World J Gastroenterol. 2007;13(30):4122–5.
- 15. Woo Y, Son T, Song K, Okumura N, Hu Y, Cho GS, et al A Novel Prediction Model of Prognosis After Gastrectomy for Gastric Carcinoma: Development and Validation Using Asian Databases. Ann Surg. 2016;264(1):114–20.
- 16. Chon HJ, Hyung WJ, Kim C, Park S, Kim JH, Park CH, et al Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg. 2017;265(5):946–53.
- 17. Nie RC, Yuan SQ, Li YF, Chen YM, Chen XJ, Zhu BY, et al Clinicopathological Characteristics and Prognostic Value of Signet Ring Cells in Gastric Carcinoma: A Meta-Analysis. J Cancer. 2017;8(17):3396–404.
- 18. Rona KA, Schwameis K, Zehetner J, Samakar K, Green K, Samaan J, et al Gastric cancer in the young: an advanced disease with poor prognostic features. J Surg Oncol. 2017;115(4):371–5.
- 19. Komatsu S, Ichikawa D, Miyamae M, Shimizu H, Konishi H, Shiozaki A, et al Histological mixed-type as an independent prognostic factor in stage I gastric carcinoma. World J Gastroenterol. 2015;21(2):549–55.
- 20. Choi JK, Park YS, Jung DH, Son SY, Ahn SH, Park DJ, et al Clinical Relevance of the Tumor Location-Modified Lauren Classification System of Gastric Cancer. J Gastric Cancer. 2015;15(3):183–90.
- 21. Kim IH, Park SS, Lee CM, Kim MC, Kwon IK, Min JS, et al Efficacy of Adjuvant S-1 Versus XELOX Chemotherapy for Patients with Gastric Cancer After D2 Lymph Node Dissection: A Retrospective, Multi-Center Observational Study. Ann Surg Oncol. 2018;25(5):1176–83.
- 22. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et alFLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
- 23. Biffi R, Botteri E, Cenciarelli S, et al Impact on survival of the number of lymph nodes removed in patients with node-negative gastric cancer submitted to extended lymph node dissection. Eur J Surg Oncol, 2011, 37(4): 0-311.
- 24. Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, et al Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24(2):486–93.
- 25. Kim J, Cheong JH, Hyung WJ, Shen J, Choi SH, Noh SH. Predictors of long-term survival in pN3 gastric cancer patients. J Surg Oncol. 2004;88(1):9–13.
- 26. Feng JF, Huang Y, Liu J, Liu H, Sheng HY, Wei WT, et al Risk factors for No. 12p and No. 12b lymph node metastases in advanced gastric cancer in China. Ups J Med Sci. 2013;118(1):9–15.
- 27. Guo DJ, Yang K, Zhang WH, Chen XL, Chen XZ, Zhang B, et al Prognostic Value of Metastatic No.8p LNs in Patients with Gastric Cancer. Gastroenterol Res Pract. 2015;2015:937682.
- 28. Yang K, Chen HN, Liu K, Zhang WH, Chen XZ, Chen XL, et al The survival benefit and safety of No. 12a lymphadenectomy for gastric cancer patients with distal or total gastrectomy. Oncotarget. 2016;7(14):18750–62.
- 29. Papachristou DN, Fortner JG. Adenocarcinoma of the gastric cardia. The choice of gastrectomy. Ann Surg. 1980;192(1):58–64.




