Introduction
Finfish aquaculture in freshwater and marine environments is continuously expanding globally (FAO, ) and the potential for a substantial further increase is well documented in the marine environment (Gentry et al., ). Freshwater aquaculture of fish dominates at present in Asia and particularly in China and Indonesia, but expansion of this branch in Europe and the Americas is possible through application of recirculation technology, which receives attention and increased investment capital. The aquaculture industry is supplying fish products for human consumption to the same extent as capture fisheries, and new fish species for domestication are still being selected by the industry (FAO, ). The challenge faced by all aquacultured species, classical and novel, is the range of pathogens associated with each new fish type. A fish host in its natural environment carries a series of more or less specific parasites (specialists and generalists) (Woo et al., ). Even if disease-free fish are used for stocking, disease problems may arise in a fish production system, provided the farm becomes exposed to pathogens (water intake from external sites). Parasite stages from wild fish populations can infect and propagate on the aquacultured fish (spill-over from the environment). As some of these organisms show a marked ability to propagate in aquaculture settings, they may elicit disease as infection intensities increase in the confined aquaculture environment. This will challenge the health and welfare of the fish and the economy of the aquaculture enterprises (Shinn et al., ). The risk of transmission of parasites from the aquaculture enterprises to wild fish stocks (back-spill) adds to the need for initiation of control programmes in order to protect the original endemic fish populations. Chemotherapeutants and medicines may be the farmer's first and convenient choice but mechanical, biological, immuno-prophylactic and genetic control methods are available as sustainable solutions. The present study outlines control possibilities for various parasitic groups of importance including oomycetes (Saprolegnia), protozoans (amoebae, flagellates, ciliates) and metazoans (myxozoans, monogeneans, digeneans, cestodes, nematodes, crustaceans) and advocates for an integrated control strategy due to the remarkable adaptivity of parasites. As background for the assessments and recommendations, a selection of relevant published scientific articles were included. Those studies are elaborating on various methods used to control parasitic infections in aquacultured fish with focus on chemotherapeutants, biocides, herbal extracts, medicines, mechanical methods and immunoprophylactic methods including vaccination. Combination of the methodologies may be considered if an integrated control strategy is to be implemented. The different legislation on usage of biocides and medicines in different countries may complicate their application as many of the compounds may be licensed in some countries but not in others.
Chemotherapeutants and biocides
A series of chemicals with a problematic toxicity profile are well known in aquaculture suffering from ectoparasitic infections. The organic dye malachite green was previously applied in even low concentrations for elimination of oomycetes (e.g. Saprolegnia) from fish eggs and fish larvae. It also effectively kills parasitic ciliates such as Ichthyophthirius multifiliis and flagellates such as Ichthyobodo, Piscinoodinium and Amyloodinium. However, concerns on the toxicity of malachite green were raised early (Alderman, ). Studies have shown that the compound and its metabolite leucomalachite green are carcinogenic and genotoxic (EFSA, ). Following the ban of malachite green several decades ago, other chemicals with some, but lower, efficacy were used in increasing amounts (Rintamäki et al., ). A range of insecticides (malathion and parathion) were previously used to eradicate crustacean parasites (Kabata, ), but the environmental issues, including toxicity to fish and workers, limit their application.
Sodium chloride and freshwater
Ectoparasites on freshwater fish may be eliminated by immersion of the infected host into high NaCl concentrations. Similarly, marine parasites succumb when exposed to freshwater dependent on their ability to adjust to the change of salinity. Parasites as free-living invertebrates may show different tolerance to changing salinities. Thus, some are euryhaline and others stenohaline. The osmotic stress induced by a change of salinity may kill a range of protozoans (amoebae, flagellates, ciliates) and metazoans (monogeneans). Freshwater treatments are regularly applied to reduce populations of marine amoebae such as Neoparamoeba perurans on gills causing amoebic gill disease (AGD) in maricultured Atlantic salmon (Nowak, ). The treatment of white spot disease caused by trophonts of the freshwater ciliate Ichthyopthirius multifiliis is more complicated. The parasite is in principle not an ectoparasite, due to its location in the epidermis, where it is covered by a hyperplasic epithelium. It is therefore protected against osmotic stress in the host tissue. In order to eliminate this parasite in a fish farm system, the free-living stages (tomonts, tomocysts, theronts) must be targeted. This may be achieved by sustaining a high (10 ppt) concentration over 10 days at temperatures over 20°C, whereby all trophonts in the fish surface will have sufficient time to escape into the fish tank water. The high salinity will prevent development of the tomont, via the tomocyst stage, into infective theronts, whereby the life cycle is broken and the parasite population exhausted (Li and Buchmann, ). The corresponding marine species Cryptocaryon irritans may be controlled by a similar strategy but by use of seawater diluted (up to 1:3) with distilled water (Cheung et al., ).
Formalin and chloramine T
Administration of formalin directly to fish tank water containing live infected fish is currently used in conventional farms and even in some recirculated systems (Madsen et al., ; Noga, ). Such bath treatments with the chemical in concentrations around 20–50 mg L−1 remove epibionts (sessile ciliates, flagellates, amoebae) (Buchmann and Bresciani, ; Noga, ) including Amyloodinium (Noga, ), Ichthyobodo (Jaafar et al., ) from fish surfaces, monogeneans from fish skin (Buchmann and Kristensson, ), gills (Buchmann, ) and kill infective free-living stages of e.g. Ichthyophthirius and Diplostomum (Larsen et al., ) in the fish tank water. In addition, it reduces the bacterial concentration and the infective stages of various pathogens. The bath treatment initiates a stress response in the fish, which can be measured as a surge of plasma cortisol (Jørgensen and Buchmann, ) and a general upregulation of proinflammatory cytokines in skin and gills (Mathiessen et al., ). As the compound is allergenic and carcinogenic it is considered a human health hazard. Chloramine T has been widely used as bath treatment against similar ectoparasites but due to lack of approval by authorities the application is restrained (Lasee, ).
Copper sulphate and potassium permanganate, iron and organic acids
Another widely used compound is copper sulphate with corresponding lethal effects on ectoparasites and or external infective stages (Lasee, ; Noga, ). It has documented toxic effects on Ichthyophthirius, Ichthyobodo, Amyloodinium and the crustacean parasite Argulus. Potassium permanganate has been used for similar purposes (Lasee, ; Straus and Griffin, ; Noga, ). Environmental concerns due to its effect on free-living organisms, including algae, may constrain approval, licensing and thereby usage in aquaculture facilities.
Hydrogen peroxide (H2O2) and H2O2-releasing compounds
Hydrogen peroxide, sodium percarbonate and peracetic acid are potent oxidizing agents, which are widely applied in aquaculture as replacement for malachite green and formalin (Rach et al., ; Meinelt et al., ; Straus and Meinelt, ; Bruzio and Buchmann, ; Jaafar et al., ). The compounds are used for bathing of infected fish and interact with and effectively kill various ectoparasites and external stages such as theronts of Ichthyophthirius and Ichthyobodo necator. It is also applied in Mediterranean mariculture enterprises, including seabream aquacultures, suffering from gill infections caused by the monogenean Sparicotyle chrysophrii (Sitjà-Bobadilla et al., ). Further, hydrogen peroxide has been used for treatment of Japanese tiger puffer (Tagifugu rubriceps) suffering from infections of the branchial cavity wall with the diclidophorid gill monogenean Heterobothrium okamotoi (Ogawa and Yokoyama, ). The compound has also been widely applied to remove salmon lice from the surface of Atlantic salmon, but efficacy has decreased over time due to selection of partly H2O2-resistant parasite strains (Helgesen et al., ).
Plant extracts
Extensive work has been conducted on the usage of plant extracts to control fish diseases, including those caused by bacterial infections (Zheng et al., ; Diler et al., ) and ectoparasites (Madsen et al., ; Tedesco et al., ). The volatile molecules in Allium, Thymus, Origanum and Coriander were found to have a short-term effect both in vitro and in vivo when screened for effects against Ichthyophthirius in rainbow trout (Mathiessen et al., ), and correspondingly Origanum extracts were found lethal to Trichodina and Ichthyobodo (Mizuno et al., ). In feed application of Chinese herbal medicines such as ginger (Zingiber officinale) also showed a significant reducing effect on I. multifiliis infection in grasscarp (Lin et al., ). The number of potential parasiticides in plants is high and a range of studies have documented effects of 18 compounds against the parasitic dinoflagellate Amyloodinium ocellatum (Tedesco et al., ). Several others may have a potential for future licensing, but unfortunately a large part of the tested substances exhibited toxic effects in cell cultures. Functional feeds (containing plant extracts, organic acids and yeast constituents) for gilthead seabream were shown to partly counteract the pathology induced by Enteromyxum leei (Palenzuela et al., ). Besides the direct toxic effect of the molecules on the parasites and a possible immunostimulatory effect on the host (Lin et al., ; Mathiessen et al., ), it is worthwhile to consider alternative mechanisms exerted by the herbal extracts, as they may disrupt the host-seeking behaviour of certain parasites and thereby prevent infections (O'Shea et al., ).
Bacterial surfactants
Unicellular parasites, such as ciliates, are sensitive to a surfactant released by the bacterium Pseudomonas H6. In vitro exposure of Ichthyophthirius theronts, tomonts and tomocysts demonstrated a full lethal effect of the compound, even when used in low concentrations (Al-Jubury et al., ). Follow-up in vivo work showed that the compound in a concentration of 10 mg L−1 in a fish tank with a high concentration of infective theronts effectively prevented infection of rainbow trout (Li et al., ). The low toxic effect of the surfactant on trout (Mathiessen et al., ) and to other ecosystem organisms (cyanobacteria, green algae, crustaceans and zebrafish) (Korbut et al., ) suggests that the product should be further assessed for a possible future application as a parasiticide in aquaculture.
Medicines
The classical approach to parasite control is to apply various medicines as antiparasitic agents (Picon-Camacho et al., ), but a set of rules and legislation must be observed when parasitic infections in fish are to be treated with medicines. This applies for both preliminary investigational and validation studies before licensing and when administrating the licensed products (Sommerville et al., ). Before initiating treatments at farm level, a specific diagnosis should be stated and a prescription made by a veterinarian. The drug should be licensed in the particular country in which the treatment is planned. Several medicines with a known antiparasitic effect have been banned in animal production of various reasons.
Nitroimidazoles
The group of drugs banned include, e.g. for the group of nitro-imidazoles, such as metronidazole, secnidazole and dimetridazole, although they are highly effective against flagellates (Spironucleus vortens) (Sangmaneedet and Smith, ) and ciliates (Ichthyophthirius) (Tokşen and Nemli, ). Usage of these drugs for production animals was banned in the European community for decades due to lack of needed documentation (safety, residual levels). Drugs which are licensed for one host species may in several countries be applied also to treat corresponding infections in other hosts, provided that sufficient documentation for efficacy against the disease and low toxicity to the host are available. In that case the prescription is given according to cascade rules.
Anticoccidials
Toltrazuril (brand name Baycox®) is an anticoccidial which was mentioned as a parasiticide by Schmahl et al. () following experimental in vitro studies with various ciliate parasites ranging from Ichthyophtirius to Apiosoma and Trichodina. In vivo work documented a preventive effect, when used in-feed, against Ichthyophthirius as well (Jaafar and Buchmann, ). Trophonts in the skin were not affected by treatment. The extended half-life in the environment makes the usage questionable from an environmental point of view. Other anticoccidials such as amprolium and salinomycin may show effect against the myxozoan E. leei in maricultured bream (Golomazou et al., ), although the exact mode of action is still to be determined.
Organophosphates
The aquaculture industry initiated the usage of various types of organophosphates including metrifonate, dichlorvos and azamethiphos at early time points. The mode of action is the inhibition of the acetylcholinesterase in the parasites, whereby the worms get paralysed. Low concentrations (<1 mg L−1) have been shown to limit infections with monogeneans such as Dactylogyrus in cyprinids (Kabata, ), Pseudodactylogyrus in eels (Chan and Wu, ), crustacean parasites (e.g. Lernaea and Argulus) in cyprinids and Lepeophtheirus in salmon farming. Early warnings against development of anthelmintic resistance in monogeneans were placed by Goven et al. () and the extensive usage of the compounds (such as azamethiphos and others) in salmonid mariculture led to fast and well-documented selection of resistant strains of salmon lice (Kaur et al., ).
Pyrethroids
Natural extracts of the plant Chrysanthemum containing pyrethroids have a strong effect on crustacean parasites such as Argulus and were used in classical Chinese fish farming as a parasiticide (Kabata, ). Pyrethroids, such as the compound deltamethrin, were also applied against salmon lice in salmonid mariculture but continuous administration induces selection of resistant strains (Bakke et al., ). The toxicity of the compound to fish calls for precaution when applying these substances.
Avermectins
The salmon industry has suffered from significant infections by salmon lice Lepeophtheirus salmonis since the early start in the late 1970s and early 1980s. The usage of hydrogen peroxide and organophosphates such as azamethiphos and metrifonate (brand names Neguvon and Nuvan) was preferred in the first decades of Norwegian mariculture. The emamectin benzoate (an avermectin product) was introduced in the late 1990s and shown highly effective as convenient in-feed treatment (Stone et al., ). Other crustacean parasites such as Argulus could be controlled in a corresponding way (Hakalahti et al., ). This accelerated its use until widespread drug resistance appeared in salmon lice (Lees et al., ), whereafter the industry turned to other ways of control (cleaner fish, mechanical removal, flushing with high-temperature water).
Benzimidazoles
Mebendazole belongs to the benzimidazole group, which has been used in human and veterinary medicine for decades. A solution of the compound was shown by Szekely and Molnar () to exert a strong and effective effect on the gill monogenean Pseudodactylogyrus parasitizing the European eel Anguilla anguilla. Following toxicological studies in the laboratory, it was also found effective as a bath in large-scale settings in recirculated eel farms (Buchmann and Bjerregaard, ). It was then regularly and extensively used in the aquaculture industry for years despite laboratory experiments warned about the risk for development of anthelmintic resistance (Buchmann et al., ). Consequently after 6 years, it could be demonstrated that a high degree of anthelmintic resistance occurred at farm level (Waller and Buchmann, ). Other benzimidazoles such as flubendazole and albendazole showed effects as well but due to their common mode of action (binding to tubulin monomers) cross resistance is expected to occur due to several common structures of the molecules. Problematic monogenean (Heterobothrium okamatoi) infections of maricultured tiger puffer in Japanese waters were previously treated by hydrogen peroxide bathing (Ogawa and Yokoyama, ), but a new compound, the pro-benzimidazole febantel, has been the drug of choice for the last decades (Hirazawa et al., ; Kimura et al., ). Elimination of external parasites, such as monogeneans, by use of anthelmintics is less complicated because the affected parasites are released from the host surface. However, endoparasites represent another problem. Benzimidazoles have seen a wide application in treatment of livestock nematode infections and fish nematodes such as Anguillicoloides (swimbladder nematode) in eels are susceptible as well. However, when treating fish with large nematode burdens in internal organs (such as the swim bladder) special attention should be placed on the risk of excessive antigen liberation from dying worms. The exposure of the host to high nematode antigen concentrations (in organs or systemically) may lead to an exacerbated immuno-pathological reaction.
Praziquantel
Already four decades ago the anthelmintic praziquantel was found highly effective against the trematode Schistosoma and subsequently it was introduced in various aquaculture settings targeting monogeneans (Schmahl and Mehlhorn, ; Sitjà-Bobadilla et al., ), trematodes such as Diplostomum eyeflukes (Bylund and Sumari, ) and cestodes such as Bothriocephalus (Pool et al., ). The compound is still being applied although lower sensitivities of various parasite species have been reported.
Antibiotics (fumagillin)
Infections of fish with myxozoans are a major problem in both marine and freshwater. The infections are not readily treated but an antibiotic, termed fumagillin as it is isolated from Aspergillus fumigatus, has been shown to prevent development and cyst formation in the fish. Documentation was provided for Tetracapsuloides bryosalmonae (PKD agent) in salmonids (Hedrick et al., ), Myxidium giardi in eel (Szekely et al., ), Myxobolus spp. in common carp (Buchmann et al., ) and E. leei in maricultured sharpsnout bream (Golomazou et al., ).
Chitin synthesis inhibitors
Arthropods, such as insects and crustaceans (including parasites such as parasitic copepods, isopods and branchiurans), perform several moults during their life cycle, which leaves them vulnerable to compounds inhibiting their formation of a new exoskeleton made of chitin. The chitin synthesis inhibitors comprise several compounds, which have been successfully used against salmon lice infections in Atlantic salmon in Norwegian mariculture and against the isopod Ceratothoa oestroides infecting seabass, seabream and meagre in Mediterranean mariculture (Bouboulis et al., ; Colak et al., ). Diflubenzuron, hexaflumuron, lufenuron and teflubenzuron all inhibited the transition of the salmon louse from the nauplius to the copepodid stage. The inhibition was associated with a decreased expression of the chitin synthase 1 gene in hexaflumuron and diflubenzuron-treated larvae (Harðardottir et al., ). Environmental concerns with regard to free-living invertebrates and vertebrates may limit the use of the drugs.
Mechanical control
Mechanical filters
The most direct way to limit development of a parasite infection in a host population is to block the life cycle. For certain parasites with free-living infective stages this can be achieved by continuous mechanical filtration of fish tank water. Various mesh sizes of filter screens may be selected to fit the size of the parasite. Thus, tomonts of Ichthyophthirius released from the skin of fish generally have a diameter of several hundred micrometres and will be trapped by filters, even with a mesh size of 80 μm (Heinecke and Buchmann, ). Whenever a tomont is trapped by the filter and removed from the system, then the cyst formation is prevented, and thereby also production of up to 1000 infective theronts within the next 36 h, dependent on temperature. Infective cercariae of eye flukes may correspondingly be trapped by use of mechanical filters (Larsen et al., ). Filtration of tank water in eel farms using 40 μm filters is able to remove eggs and oncomiracidia of the gill parasitic monogenean Pseudodactylogyrus anguillae and Pseudodactylogyrus bini (Buchmann, ).
Parasite egg traps
The branchiuran parasite Argulus reproduces by laying egg clusters on submerged objects including aquatic plants, branches and roots. Following hatching of Argulus eggs, the larvae develop into the infective stage and attach to the fish. This reproductive strategy can be utilized for control. Regular immersion into an infected pond of wooden slats, lattices and bundles of branches to which the female parasite attaches egg clusters provides an opportunity to kill the eggs. This is achieved simply by removing these egg traps from the ponds with few days intervals. New traps are replacing the recovered ones and will be used for parasite oviposition. In this way the reproductive potential of the parasites can be, at least partly, exhausted leading to a decreased infection pressure in the ponds (Kabata, ). Submerging boards of various materials into water bodies with problematic Argulus foliaceus infections on rainbow trout have been used to reduce the infection level of Argulus in rainbow trout lakes (Gault et al., ). Egg clusters could be harvested regularly when recovering the boards and pulling them ashore. Thereby the overall infection pressure fell and the prevalence and mean intensity decreased 6- to 9-fold.
Delousing management
Due to the decreasing sensitivity of salmon lice to the different biocides, chemotherapeutants and medicines, which occurred after extensive usage in mariculture farms, alternative control methods had to be developed. These included mechanical removal by heat treatment, brushing or flushing with freshwater. The technique necessitates capture and handling of large salmon, which challenges the health and welfare of the fish (Østevik et al., ). Other approaches in action are based on laser technology targeting salmon lice on the fish surface. Automated camera systems placed in the water are able to scan passing fish in the netpen and if a salmon louse is detected the laser entity emits a pulse of high energy light towards the louse aiming at killing the parasite in situ. Although potentially lethal to the louse, recent controlled full-scale tests could not document a decrease in the mean number of parasites in laser-exposed netpens (Bui et al., ).
Farm and netpen construction
New design of netpens applies the use of barriers preventing entrance of infective parasite stages into the section with fish. The upper water layers are preferred by the infective copepodids of the salmon louse. In salmon, mariculture skirts or tarpaulin may be placed around the upper part of the netcages in order to reduce contact between fish and parasites and thereby infections. Thus, the so-called snorkel netcage farms have been developed in order to minimize the attachment of sealice copepodids on maricultured Atlantic salmon. The fish are kept in submerged netpen compartments in the deeper water layers, zones which have reduced abundance of infective louse stages due to the surface-seeking behaviour of copepodids. A lower mean intensity of infection was observed in these cages (Geitung et al., ).
Intermediate host control
Eyefluke infections in fish are caused by infective cercariae, released from intermediate host snails, penetrating the surfaces of fish skin, fins or gills (Duan et al., ). If these cercariae cannot be eliminated by chemicals or mechanical filtration of water (Larsen et al., ) it is possible to remove the intermediate host snails simply by collecting snails from ponds. As each snail may produce 58 000 cercariae per day this procedure may effectively limit the infection pressure in a pond (Lyholt and Buchmann, ).
Biological control
Cleaner fish
Natural marine and freshwater ecosystems exhibit a wealth of symbiotic relationships between fish infected with ectoparasites. In tropical fish farming, mosquito fish Gambusia feed on the branchiuran parasite Argulus during their free-swimming activity (Kabata, ) and smaller fish can easily recognize ectoparasites on other often larger fish and pick them of the host skin (Bjordal, ; Cowell et al., ). This basic biological function is being applied by the industry by stocking salmon netpens with cleanerfish. Various species of wrasse have been applied during the latest three decades for removal of salmon lice from salmon skin (Bjordal, ; Groner et al., ; Imsland et al., ). Their effect is low during wintertime whereas another cleanerfish, lumpsucker Cyclopterus lumpus, has a superior performance at low temperatures. A huge industry has been established in order to produce lumpsucker, a species with appetite for salmon louse attached to salmon skin (Groner et al., ; Imsland et al., ). Even this sustainable approach is challenged by the high adaptability of salmon lice. Forms with a lower degree of pigmentation, and thereby a lower chance of being recognized and eaten by cleaner fish, have appeared. Both environmental and genetic factors may influence this change of pigmentation (Hamre et al., ), but these less visible lice are likely to decrease the efficacy of cleaner fish. Various fish species predating on snails (Ben-Ami and Heller, ) may be considered a supplement for control of digenean parasites using snails as intermediate hosts. By eliminating snails by predation these fish may contribute to a lowered infection level.
Cleaner invertebrates
In addition to cleaner fish removing ectoparasites from the surface of infected production fish, a series of other solutions, based on predation by invertebrates, for parasite control exist. The filtration of huge water masses by blue mussels Mytilus edulis can be used for trapping the pelagic larval stages (copepodids) of salmon lice L. salmonis (Bartsch et al., ). Free-living copepods such as Cyclops predate on fish parasitizing Diplostomum cercariae (Bulaev, ) and oncomiracidia of Pseudodactylogyrus monogeneans (Buchmann, ). Likewise free-living turbellarians (Stephanostomum sp.) ingest freshly delivered eggs of Pseudodactylogyrus whereby the infection level for fish decreases (Buchmann, ).
Immunological control
Immunostimulants
Stimulation of the immune system of the teleost host by adding various immunestimulants to the feed is a strategy applied by aquaculturists to a wide extent. Slight decreases of infection levels may be recorded following this type of feeding both with regard to protozoans such as Ichthyophthirius (Xueqin et al., ) and metazoans, such as L. salmonis (Poley et al., ). Effects of caprylic acid in combination with iron and mannan (a potential immunostimulant) in feed for seabream in Mediterranean mariculture against monogenean infections (Sparicotyle chrysophrii) were recorded by Rigos et al. (), but the exact mode of action needs to be investigated. However, the efficacy of in-feed immunostimulants for protection of fish against pathogens is generally very low when compared to the effect of vaccination.
Vaccination
Today vaccination against both bacterial and viral diseases has proven to be the most sustainable ways to control fish disease in aquaculture enterprises. In Europe alone about 1.3 billion fish are successfully vaccinated annually against various infective diseases (Midtlyng, ). The marked immune responses established in fish, when infected by various protozoan and metazoan parasites, may lead to some level of protective immunity in fish surviving a natural infection. This was documented for the parasitic ciliates Ichthyophthirius (Buschkiel, ; Bauer, ; Hines and Spira, ; Sigh and Buchmann, ; Alishahi and Buchmann, ) and Philasterides (Lamas et al., ), for the flagellates Trypanosoma (Woo, ), Cryptobia (Jones and Woo, ) and Ichthyobodo (Chettri et al., ), for monogeneans such as Gyrodactylus (Lindenstrøm and Buchmann, ), Pseudodactylogyrus (Slotved and Buchmann, 1993) and the crustacean parasite Lernaea (Woo and Shariff, ). This suggests the existence of a potential for development of antiparasitic fish vaccines (Jørgensen et al., ), but up until now no such products are licensed for use in commercial aquaculture. Immunological protection of fish against various parasites is likely to be based on a plethora of cellular and humoral (innate and adaptive) elements, which are not so easily induced by a vaccine. So, although no antiparasitic vaccines are available for fish, it may be worthwhile to apply controlled (low to moderate) parasite infections in order to induce a protective response, which may supplement other control strategies. This response induced by a natural infection is likely to include the relevant immune reactions needed to control, at least partly, the host against reinfection.
Parasitic disease resilience and gut microbiota
The interface between mucosal immunity, gut microbiota and resistance towards parasites has been explored with some success. Stimulation of the gut microbiota by functional feed additives, such as sodium butyrate, may be a way to at least partly control infections with the myxozoan E. leei in maricultured seabream (Piazzon et al., ). It may also be speculated if feed additives such as caprylic acid and mannan exert their effects on the gill monogenean S. chrysophryi through a general systemic immune stimulation in seabream (Rigos et al., ).
Genetic control through breeding
The ability to resist a parasitic infection is genetically determined, which has been indicated for Ichthyophthirius and Myxobolus infections in rainbow trout (Hedrick et al., ; Avila et al., ). Breeding towards more disease-resistant fish is a possibility, and marker-assisted selective breeding is a tool which has been increasingly used in breeding programmes. Selective breeding of fish carrying certain desirable traits has been in use for decades also in fish aquaculture. The classical approach often takes several years before results are seen, because the generation time of fish can be several years. Several studies have discovered quantitative trait loci (QTL) for viral, bacterial and parasitic diseases. This was demonstrated for infectious pancreatic necrosis virus (Houston et al., ; Moen et al., ) and salmonid alpha virus (Aslam et al., ) in Atlantic salmon and for viral haemorrhagic septicaemia virus resistance in rainbow trout (Verrier et al., ). Studies have also described QTL associated with resistance in salmonids to bacterial infections caused by Piscirickettsia salmonis (Correa et al., ), Flavobacterium psychrophilum (Wiens et al., ; Vallejo et al., ) and Vibrio anguillarum (Du et al., ; Wang et al., ; Shao et al., ; Karami et al., ). In addition, the approach is useful for parasite–host systems as well. QTL for resistance towards AGD were described in Atlantic salmon (Robledo et al., ) and for the scuticociliate Philasterides dicentrachi infecting turbot (Rodriguez-Ramilo et al., ). QTL for resistance in rainbow trout against Myxobolus cerebralis was studied by Hedrick et al. () and later by Baerwald et al. (). Investigation of single-nucleotide polymorphism (SNP) markers indicated that some genes associated with resistance towards I. multifiliis are located on rainbow trout chromosomes 16 and 17 (Jaafar et al., ). Innate response genes in the Atlantic salmon were targeted by Gilbey et al. () focusing on gyrodactylid monogeneans (Gyrodactylus salaris). Likewise, Gharbi et al. () and Robledo et al. () searched for genes associated with resistance towards salmon lice in this host. Ozaki et al. () found corresponding host–parasite associations for the capsalid monogenean Benedenia seriolae infecting yellowtail Seriola quinqueradiata. Novel typing technology applying markers makes it easier to conduct genotyping. A microarray comprising 57 501 markers (SNP) was used to locate genes encoding resistance to V. anguillarum on chromosome 21 (Omy 21) (Karami et al., ), and genes associated with Ichthyophthirius resistance [chromosomes 16 and 17 (Omy 16 and 17)] (Jaafar et al., ). Genotyped breeders (females and males) carrying the SNPs associated with the favourable trait can easily be selected and used for production of a new generation of trout with a higher innate resistance to infection (Buchmann et al., ). The basis for this innate and heritable protection may be partly associated with immune factors in the host. However, it cannot be excluded that other elements, including chemoattraction of the parasite to the host, may be involved in better performance of QTL fish.
Integrated control
Parasites are in general highly adaptable to environmental changes and even a documented control method may in a few years show inferior. An example from the search on compounds with lethal effects on salmon lice is the successful introduction, documentation and victory of the avermectin emamectin benzoate against L. salmonis infections. However, its use decreased due to rapid selection of drug-resistant parasite strains. Similar processes apply for organophosphates and pyrethroids. Parasites possess a remarkable ability to adjust to environmental changes as strains with resistance to new conditions are readily selected. In order to secure the fish production from massive infestations of parasites it is worthwhile to introduce integrated control strategies involving several antiparasitic approaches. At present, control of Ichthyophthirius infections in freshwater trout farms involves mechanical filtration with micro-sieves (removing tomonts from the water phase), regular addition to fish pond water of biocides/auxiliary substances (hydrogen peroxide, peracetic acid or sodium percarbonate eliminating infective theronts), tolerance of a low initial parasite infection (which induces a relative and partly protective immune response) and use of breeds with an elevated level of natural resistance to infection (Jaafar et al., ; Buchmann et al., ).
Discussion
Parasitism is one of the most successful life forms on earth. The use of a host for transport, forage area and breeding zone is smart and has therefore been selected by the majority of existing species through evolution. Most free-living host species carry more or less specific parasites and parasites carry in most cases even hyperparasites. Although a parasite seeks to remain undetected on or in the host in order to avoid killing from various host immune element infections they may eventually be confronted with marked host responses. The parasite population may reach levels which elicit injuries and induce pathological and humoral/cellular reactions which expel the parasite from the host. During this process immunosuppressive molecules are often produced by the parasite in order to minimize the host response but this may weaken the host even more and make it susceptible to infections by bacterial and viral pathogens (Kamiya et al., ). This applies for fish parasites as well. Aquaculture of teleosts is generally based on keeping fish in confined environments ranging from earth or concrete ponds or raceways (Fig. 1), via netpens in lakes and marine areas (Fig. 2) to in-house recirculated farms on land (Fig. 3). In all systems parasite infections may be so severe that profitability of the enterprise become endangered due to losses of fish (mortality) or decreased prices from fish products of lower quality (Shinn et al., ). Aquaculturists must necessarily instigate control measurements, and the present report has briefly introduced various options including chemical, medical, mechanical, genetic and biological control methods. In mariculture it is possible to prevent infection by anisakid nematodes by sequestering fish in e.g. netpens and feed them only heat-treated feed, whereby live nematode larvae cannot reach the fish (Fioravanti et al., ). In addition, prevention of any introduction of pathogens, including parasites, may be achieved in closed high technology systems with recirculation of water. Disinfection of the entire production system after each production cycle, and combined with introduction only of certified pathogen free and disinfected eggs, will allow running of a disease-free system (Xueqin et al., ). The use of sterilizing measures such as UV-irradiation (Gratzek et al., ) and ozonization is able to support such a system. However, the costs associated with such a management practice are high and may challenge profitability. Most systems are therefore facing parasite-induced problems. Experience shows that only one control method is likely to fail at a certain time. This encourages the implementation of an integrated control strategy seeking to combat parasites through multiple targets (Fig. 4). The classical and convenient way to address a newly diagnosed disease is to apply chemotherapeutants and medicines but as mentioned above, the impressive adaptability of parasites may leave these approaches less effective, or near useless, within a few years of intensive usage (Helgesen et al., ; Kaur et al., ). Resistance to treatments is easily developed, and increase of the therapeutic dosage is not always possible due to the toxicity of parasiticides to the host organisms (Tedesco et al., ). In-feed use of immunostimulants for the fish may elevate the resistance to infection marginally but cannot alone sustain health in any production (Akhter et al., ; Mathiessen et al., ). Vaccination of fish against bacterial and viral diseases is to a wide extent applicable as documented by a catalogue of vaccine products licensed and currently being used (Midtlyng, ). However, effective vaccines against fish parasites are not readily produced (Jørgensen et al., ), despite available documentation of occurrence of protective immune responses in fish against both protozoan (Woo, ; Sigh and Buchmann, ) and metazoan parasites (Woo and Shariff, ; Lindenstrøm and Buchmann, ) following natural infections. More or less controlled infections of farmed fish at a sufficiently low level may induce a level of immunity, which can contribute to an acceptable presence of parasites in farms. The limited number of control methods based on chemicals, drugs and vaccines has forced the industry to develop alternative mechanical techniques, some of which have been used in classical but more primitive fish farming. In this context the use of cleaner fish, which clearly reduces the load of ectoparasites on farmed fish (Bjordal, ; Cowell et al., ), is still an option despite the selection of less pigmented and hardly visible salmon lice is known to challenge their performance (Hamre et al., ). The exhaustion of the reproductive potential of a population of Argulus by regularly harvesting their egg clusters on submerged material is laborious but possible (Kabata, ; Gault et al., ). Correspondingly, removal of parasite stages (ciliates, monogenean eggs, digenean larvae, crustacean larvae) by mechanical filtration (Larsen et al., ; Heinecke and Buchmann, ) or biological mussel filtration (Bartsch et al., ) has also a potential, although absolute control is not achieved. Another promising strategy is to apply selective breeding of fish with a certain level of natural resistance to infection (Jaafar et al., ; Avila et al., ). The adaptability of parasites to any environmental disturbance or challenge makes it necessary to combine the different control systems in order to reduce the selective pressure for occurrence of avoidance mechanisms in parasites. Such an integrated approach has been installed in many types of fish culture systems. In freshwater trout farms mechanical filters (mesh size 40 or 80 μ) have been installed for removal of particles (Fig. 5). This will also eliminate a sub-population of the I. multifiliis tomonts floating in the water currents and thereby prevent their transformation into the sessile and attached tomocyst stage and their subsequent release of infective theronts (Heinecke and Buchmann, ). Removal of tomocysts may also be achieved through suction devices (vacuum cleaning) of fish tanks specifically designed to resist the attachment of tomocysts (Shinn et al., ). As a supplement the use of regular additions of peracetic acid (Meinelt et al., ; Bruzio and Buchmann, ), sodium percarbonate or hydrogen peroxide reduces the concentration of infective theronts (Heinecke and Buchmann, ). Formalin is still being used for the same purpose but its adverse effect on the surface epithelia of the fish may question the use as seen from fish welfare point of view (Buchmann et al., ; Mathiessen et al., ). Further, due to the carcinogenicity of formalin it is listed as a human health hazard. The recent documentation of antiparasitic effects of a natural bacterial surfactant released from the bacterium Pseudomonas H6 (Li et al., ) may add this novel compound to the list of possible control agents. As this method is not absolutely effective at the farm level, a sustained parasite infection pressure will occur. However, this may be sufficient to establish a relatively low infection of fish, which will induce a partly protective immune response. Recently, QTL for I. multifiliis resistance were described (Jaafar et al., ), validated (Buchmann et al., ) and then used for selection of parent fish with a documented better resistance. The specific markers (SNPs) are now being implemented in breeding programmes whereby the resulting new generations carry genes for natural resistance against this particular parasite. The mentioned ways to control parasite occurrence in fish farms have all at a certain time proved effective to some extent. However, the impressive adaptability of both protozoan and metazoan parasites to environmental manipulations in both freshwater and marine aquaculture facilities will in a few years result in parasite strains resisting various treatments if used repeatedly over time. It is therefore recommended to apply an integrated control strategy (Fig. 4). Alternation between control methods will limit the selective pressure for development of resistance to a particular control method. Some techniques may be applied at the same time (e.g. filtration of water to remove Ichthyophthrius tomonts and concomitant peracetic water treatment to eliminate theronts). Others may be used in alternation, which on a theoretical basis may be considered to delay selection of parasite strains with resistance to a multitude of control methods.

Fig. 1
Raceway production of freshwater rainbow trout.

Fig. 2
Mariculture farm based on netpen production of rainbow trout in the sea.

Fig. 3
In-door recirculated rainbow trout production.

Fig. 4
Diagrammatic overview of control elements to be combined into an integrated approach for prevention or treating fish parasite infections in aquacultured fish.
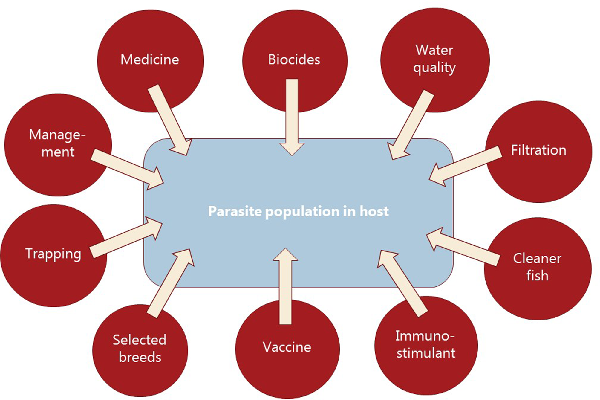
Fig. 5
Mechanical filter removing particles in a freshwater trout farm.
Conclusions and future directions
The impressive ability of parasites to adapt to environmental changes challenges an effective and lasting control of parasitoses in aquaculture settings. Even novel compounds with high efficacy may be ineffective within a few years of constant usage at farm level. It is recommended that aquaculturists should combine various methods in an integrated control strategy. Alternation between different chemical, medical, biological and mechanical control methods may delay development of resistance. Continued research into basic biology and biochemistry of the parasites can lead to novel approaches replacing old and less effective methods. Up until now no effective control method based on hyperparasitism has been documented in aquaculture settings. Hyperparasites occur in natural ecosystems and future research should show if this could be a supplementary tool in fish parasite control.
Acknowledgements
The author is indebted to Drs Moonika H. Marana and Jakob Skov for providing the photograph for Fig. 2 taken in a Danish mariculture farm.
References
- Akhter N, Wu B, Memon AM and Mohsin M (2015) Probiotics and prebiotics associated with aquaculture: a review. Fish and Shellfish Immunology 45, 733–741.
- Al-Jubury A, Lu C, Kania PW, Jørgensen LG, Liu Y, de Bruijn I, Raaijmakers J and Buchmann K (2018) Impact of Pseudomonas H6 surfactant on all external life cycle stages of the fish parasitic ciliate Ichthyophthirius multifiliis. Journal of Fish Diseases 41, 1147–1152.
- Alderman D (1985) Malachite green – a review. Journal of Fish Diseases 8, 289–298.
- Alishahi M and Buchmann K (2006) Temperature-dependent protection against Ichthyophthirius multifiliis following immunisation of rainbow trout using live theronts. Diseases of Aquatic Organisms 72, 269–273.
- Aslam ML, Robledo D, Krasnov A, Moghadam HK, Hillestad B, Houston RD, Baranski M, Boison S and Robinson NA (2020) Quantitative trait loci and genes associated with salmonid alphavirus load in Atlantic salmon: implications for pancreas disease resistance and tolerance. Scientific Reports 10, 1–15.
- Avila BW, Dana L, Winkelman DL and Fetherman ER (2022) Dual resistance to Flavobacterium psychrophilum and Myxobolus cerebralis in rainbow trout (Oncorhynchus mykiss, Walbaum). Journal of Fish Diseases 45, 801–813.
- Baerwald MR, Petersen JL, Hedrick RP, Schisler G and May B (2011) A major effect of quantitative trait locus for whirling disease resistance identified in rainbow trout (Oncorhynchus mykiss). Heredity 106, 920926.
- Bakke MJ, Agusti C, Bruusgaard JC, Sundaram AYM and Horsberg TE (2018) Deltamethrin resistance in the salmon louse, Lepeophtheirus salmonis (Krøyer): maternal inheritance and reduced apoptosis. Scientific Reports 8, 1–14. doi:
- Bartsch A, Robinson SMC, Liutkus M, Ang KP, Webb J and Pearce CM (2013) Filtration of sea louse, Lepeophtheirus salmonis, copepodids by the blue mussel, Mytilus edulis, and the Atlantic sea Scallop, Placopecten magellanicus, under different flow, light and copepodid-density regimes. Journal of Fish Diseases 36, 361–370.
- Bauer ON (1953) Immunity of fish occurring in infections with Ichthyophthirius multifiliis Fouquet, 1876. Dokla Novaia erviia 93, 377–379.
- Ben-Ami F and Heller J (2001) Biological control of aquatic pest snails by the black carp Mylopharyngodon piceus. Biological Control 22, 131–138.
- Bjordal A (1991) Wrasse as cleaner-fish for farmed salmon. Progress in Underwater Science 16, 17–28.
- Bouboulis D, Athanassopoulou F and Turpenou A (2004) Experimental treatments with diflubenzuron and deltamethrin of sea bass, Dicentrarchus labrax L., infected with the isopod, Ceratothoa oestroides. Journal of Applied Ichthyology 20, 314–317.
- Bruzio R and Buchmann K (2010) The effect of peracetic acid products on parasites causing white spot disease. Fish Farmer 6, 25–27.
- Buchmann K (1988) Epidemiology of pseudodactylogyrosis in an intensive eel-culture system. Diseases of Aquatic Organisms 5, 81–85.
- Buchmann K and Bjerregaard J (1990) Mebendazole-treatment of pseudodactylogyrosis in intensive eel-culture systems. Aquaculture 86, 139–153.
- Buchmann K and Bresciani J (1997) Parasitic infections in pond-reared rainbow trout Oncorhynchus mykiss in Denmark. Diseases of Aquatic Organisms 28, 125–138.
- Buchmann K and Kristensson RT (2003) Efficacy of sodium percarbonate and formaldehyde bath treatments against Gyrodactylus derjavini infestations of rainbow trout. North American Journal of Aquaculture 65, 25–27.
- Buchmann K, Roepstorff A and Waller PJ (1992) Experimental selection of mebendazole resistant gill parasites from the European eel. Journal of Fish Diseases 15, 393–400.
- Buchmann K, Slotved H-C and Dana D (1993) Epidemiology of gill parasites from carp (Cyprinus carpio) in Indonesia and possible control methods. Aquaculture 118, 9–21.
- Buchmann K, Bresciani J and Jappe C (2004) Effects of formalin treatments on epithelial structure and mucous cell densities in rainbow trout, Oncorhynchus mykiss (Walbaum), skin. Journal of Fish Diseases 27, 99–104.
- Buchmann K, Nielsen T, Mathiessen H, Marana MH, Duan Y, Jørgensen L, Zuo S, Karami AM and Kania PW (2022) Validation of two QTL associated with lower Ichthyophthirius multifiliis infection and delayed time-to-death in rainbow trout. Aquaculture Reports 23, 101078.
- Bui S, Geitung L, Oppedal F and Barrett LT (2020) Salmon lice survive the straight shooter: a commercial scale sea cage trial of laser delousing. Preventive Veterinary Medicine 181, 105063.
- Bulaev AI (1982) Experimental study of the elimination of cercariae by freshwater crustaceans Cyclops vicinus (order cyclopoida). Gelminty preshnovodnykh boitsenozak, Moscow, USSR. Nauka 1, 73–81, 3(In Russian).
- Buschkiel AL (1910) Beiträge zur Kenntnis dea Ichthyophthirius multifiliis Fouquet. Archiv der Protistenkunde 21, 61–102.
- Bylund G and Sumari O (1981) Laboratory tests with Droncit against diplostomiasis in rainbow trout, Salmo gairdneri Richardson. Journal of Fish Diseases 4, 259–264.
- Chan B and Wu B (1984) Studies on the pathogenicity, biology, and treatment of Pseudodactylogyrus in fish farms. Acta Zoologica Sinensia 30, 173–180. 0.0257-9.
- Chettri JK, Kuhn JA, Jafaar RM, Kania PW, Møller OS and Buchmann K (2014) Epidermal response of rainbow trout to Ichthyobodo necator: immunohistochemical and gene expression studies indicate a Th1-/Th2-like switch. Journal of Fish Diseases 37, 771–783.
- Cheung PJ, Nigrelli RF and Ruggieri GD (1979) Studies on cryptocaryoniasis in marine fish: effect of temperature and salinity on the reproductive cycle of Cryptocaryon irritans Brown, 1951. Journal of Fish Diseases 2, 93–97.
- Colak S, Kolega M, Zupan I, Saric T, Piplovic E and Mustac B (2018) Prevalence and effects of the cymothoid isopod (Ceratothoa oestroides, Risso 1816) on cultured meagre (Argyrosomus regius, Asso 1801) in the Eastern Adriatic Sea. Aquaculture Research 49, 1001–1007.
- Correa K, Lhorente JP, Lopez ME, Bassini L, Naswa S and Deeb N (2015) Genome-wide association analyses reveals two loci associated with resistance against Piscirickettsia salmonis in two Atlantic salmon (Salmo salar L.) chromosomes. BMC Genomics 16, 854.
- Cowell LE, Watanabe WO, Head WD, Grover JJ and Shenker JM (1993) Use of topical cleaner fish to control the ectoparasite on seawater cultured Florida red tilapia. Aquaculture 113, 189–192.
- Diler O, Gormez O, Diler I and Metin S (2017) Effect of oregano (Origanum onites L.) essential oil on growth, lysozyme and antioxidant activity and resistance against Lactococcus garvieae in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture Nutrition 23, 844–851.
- Du M, Chen SL, Liu Y and Yang JF (2011) MHC polymorphism and disease resistance to Vibrio anguillarum in 8 families of half-smooth tongue sole (Cynoglossus semilaevis). BMS Genetics 12, 78.
- Duan Y, Jørgensen LVG, Kania PW, Al-Jubury A, Karami AM and Buchmann K (2021) Eye fluke effects on Danish freshwater fish: field and experimental investigations. Journal of Fish Diseases 44, 1785–1798.
- EFSA (2016) Malachite green in food. European Food Safety Authority. European request EFSA-Q-2014-00815. doi:
- FAO (2020) United Nations Organisation for Food and Agriculture. The State of World Fisheries and Aquaculture (SOFIA). Rome, Italy ISBN: 978–92-5-136364-5.
- Fioravanti ML, Gustinelli A, Rigos G, Buchmann K, Caffara M, Pascual S and Pardo MA (2021) Negligible risk of zoonotic anisakid nematodes in farmed fish from European mariculture, 2016–2018. Eurosurveillance 26, pii 1900717.
- Gault NFS, Kilpatrick DJ and Stewart MT (2002) Biological control of the fish louse in a rainbow trout fishery. Journal of Fish Biology 60, 226–237.
- Geitung L, Oppedal F, Stien LH, Dempster T, Karlsbakk E, Nola V and Wright DW (2019) Snorkel sea-cage technology decreases salmon louse infestation by 75% in a full-cycle commercial test. International Journal for Parasitology 49, 843–846.
- Gentry RR, Froehlich HE, Grimm D, Kareiva P, Parke M, Rust M, Gaines SD and Halpern BS (2017) Mapping the global potential for marine aquaculture. Nature Ecology & Evolution 1, 8.
- Gharbi K, Glover KA, Stone LC, MacDonald ES, Matthews L and Grimholt U (2009) Genetic dissection of MHC-associated susceptibility to Lepeophtheirus salmonis in Atlantic salmon. BMC Genetics 10, 20.
- Gilbey J, Verspoor E, Mo TA, Sterud E, Olstad K and Hytterød S (2006) Identification of genetic markers associated with Gyrodactylus salaris resistance in Atlantic salmon Salmo salar. Diseases of Aquatic Organisms 71, 119–129.
- Golomazou E, Athanassopoulou F, Karagouni E, Vagianou S, Tsantilas H and Karamanis D (2006) Efficacy and toxicity of orally administrated anti-coccidial drug treatment on Enteromyxum leei infections in sharpsnout seabream (Diplodus puntazzo). Israeli Journal of Aquaculture Bamidgeh 58, 157–169.
- Goven BA, Gilbert JP and Gratzek JB (1980) Apparent drug resistance to the organophosphate dimethyl (2,2,2-trichloro-1-hydroxyethyl) phosphonate by the monogenetic trematodes. Journal of Wildlife Diseases 16, 343–346.
- Gratzek JB, Gilbert JP, Lohr AL, Shotts EB and Brown J (1983) Ultraviolet light control of Ichthyophthirius multifiliis Fouquet in a closed fish culture recirculation system. Journal of Fish Diseases 6, 145–153.
- Groner ML, Cox R, Gettinby G and Revie CW (2013) Use of agent based modelling to predict benefits of cleaner fish in controlling sea lice, Lepeophtheirus salmonis, infestations on farmed Atlantic salmon, Salmo salar L. Journal of Fish Diseases 36, 195–208.
- Hakalahti T, Lankinen Y and Valtonen ET (2004) Efficacy of emamectin benzoate in the control of Argulus coregoni (Crustacea: Branchiura) on rainbow trout Oncorhynchus mykiss. Diseases of Aquatic Organisms 60, 197–204.
- Hamre LA, Oldham T, Oppedal F, Nilsen F and Glover KA (2021) The potential for cleaner fish-driven evolution in the salmon louse Lepeophtheirus salmonis: genetic or environmental control of pigmentation? Ecology and Evolution 11, 7865–7878.
- Harðardottir HM, Male R, Nilsen F and Dalvin S (2019) Effects of chitin synthesis inhibitor treatment on Lepeophtheirus salmonis (Copepoda, Caligidae) larvae. PLoS ONE 14, e0222520.
- Hedrick RP, Groff JM, Foley P and McDowell T (1988) Oral administration of Fumagillin DHC protects chinook salmon Oncorhynchus tshawytscha from experimentally induced proliferative kidney disease. Diseases of Aquatic Organisms 4, 165–168.
- Hedrick RP, McDowell TS, Marty GD, Fosgate GT, Mukkatira K and Myklebust K (2003) Susceptibility of two strains of rainbow trout (one with suspected resistance to whirling disease) to Myxobolus cerebralis infection. Diseases of Aquatic Organisms 55, 37–44.
- Heinecke RD and Buchmann K (2009) Control of Ichthyophthirius multifiliis using a combination of water filtration and sodium percarbonate: dose-response studies. Aquaculture 288, 32–35.
- Helgesen KO, Aaen SM, Romstad H and Horsberg TE (2015) First report of reduced sensitivity towards hydrogen peroxide in the salmon louse Lepeophtheirus salmonis in Norway. Aquaculture Reports 1, 37–42.
- Hines RS and Spira DT (1974) Ichthyophthirius multifiliis (Fouquet) in the mirror carp, Cyprinus carpio L. V. Acquired immunity.Journal of Fish Biology 6, 373–378.
- Hirazawa N, Ohtaka T and Hata K (2000) Challenge trials on the anthelmintic effect of drugs and natural agents against the monogenean Heterobothrium okamotoi in the tiger puffer Tagifugu rubriceps. Aquaculture 188, 1–13.
- Houston RD, Haley CS, Hamilton A, Guy DR, Tinch AE and Taggart JB (2008) Major quantitative trait loci affect resistance to infectious pancreatic necrosis in Atlantic salmon (Salmo salar). Genetics 178, 1109–1115.
- Imsland AK, Reynolds P, Eliassen G, Hangstad TA, Foss A, Vikingstad E and Elvegård TA (2014) The use of lumpfish (Cyclopterus lumpus L.) to control sea lice (Lepeophtheirus salmonis Krøyer) infestations in intensively farmed Atlantic salmon (Salmo salar L.). Aquaculture 424, 18–23.
- Jaafar RM and Buchmann K (2011) Toltrazuril (Baycox vet.) in feed can reduce Ichthyophthirius multifiliis invasion of rainbow trout (Salmonidae). Acta Ichthyologica et Piscatoria 41, 63–66.
- Jaafar RM, Kuhn JA, Chettri JK and Buchmann K (2013) Comparative efficacies of sodium percarbonate, peracetic acid, and formaldehyde for control of Ichtyobodo necator – an ectoparasitic flagellate from rainbow trout. Acta Ichthyologica et Piscatoria 43, 139–143.
- Jaafar R, Ødegård J, Mathiessen H, Karami AM, Marana MH, Jørgensen LVG, Zuo S, Nielsen T, Kania PW and Buchmann K (2020) Quantitative trait loci (QTL) associated with resistance of rainbow trout Oncorhynchus mykiss against the parasitic ciliate Ichthyophthirius multifiliis. Journal of Fish Diseases 43, 1591–1602.
- Jones SRM and Woo PTK (1987) The immune response of rainbow trout, Salmo gairdneri Richardson to the haemoflagellate Crytobia salmositica Katz 1951. Journal of Fish Diseases 10, 395–402.
- Jørgensen TR and Buchmann K (2007) Stress response in rainbow trout during infection with Ichthyophthirius multifiliis and formalin bath treatment. Acta Ichthyologica et Piscatoria 37, 25–28.
- Jørgensen LVG, Sigh J, Kania WP, Holten-Andersen L, Buchmann K, Clark T, Rasmussen JS, Einer-Jensen K and Lorenzen N (2012) Approaches towards DNA vaccination against a skin ciliate parasite in fish. PLoS ONE 7, e48129.
- Kabata Z (1985) Parasites and Diseases of Fish Cultured in the Tropics. London and Philadelphia: Taylor & Francis, p. 318.
- Kamiya T, Mideo N and Alizon S (2018) Coevolution of virulence and immunosuppression in multiple infections. Journal of Evolutionary Biology 3, 95–100.
- Karami AM, Mathiessen H, Ødegård J, Marana MH, Jaafar R, Jørgensen LVG, Zuo S, Dalsgaard I, Nielsen T, Kania PW and Buchmann K (2020) Detecting a major QTL for Vibrio anguillarum resistance in rainbow trout. Frontiers in Genetics 11, 607558.
- Kaur K, Jansen PA, Aspehaug VT and Horsberg TE (2016) Phe 362Tyr in Ache: a major factor responsible for Azamethiphos resistance in Lepeophtheirus salmonis in Norway. PLoS ONE 11, e0149264.
- Kimura T, Nomura Y, Kawakami H, Itano T, Iwasaki M, Morita J and Enomoto J (2009) Field trials of febantel against gill fluke disease caused by the monogenean Heterobothrium okamotoi in cultured tiger puffer Takifugu rubripes. Fish Pathology 44, 67–71.
- Korbut R, Skjolding LM, Mathiessen H, Jaafar R, Li X, Jørgensen LVG, Kania PW, Wu B and Buchmann K (2022) Toxicity of the antiparasitic lipopeptide biosurfactant SPH6 to green algae, cyanobacteria, crustaceans and zebrafish. Aquatic Toxicology 243, 106072. (1-8).
- Lamas J, Sanmartin ML, Parama AI, Castro R and Cabaleiro S (2008) Optimization of an inactivated vaccine against a scuticociliate parasite of turbot: effect of antigen, formalin and adjuvant concentration on antibody response and protection against the pathogen. Aquaculture 278, 22–26.
- Larsen AH, Bresciani J and Buchmann K (2005) Pathogenicity of Diplostomum cercariae in rainbow trout, and alternative measures to prevent diplostomosis in fish farms. Bulletin of the European Association of Fish Pathologists 25, 332–339.
- Lasee BA (1995) Introduction to Fish Health Management, 2nd Edn. Onalaska, Wisconsin: U.S. Fish and Wildlife Service, La Crosse Fish Health Center, p. 139.
- Lees F, Baillie M, Gettinby G and Revie CW (2008) The efficacy of emamectin benzoate against infestation of Lepeophtheirus salmonis on farmed Atlantic salmon, Salmo salar, in Scotland between 2002 and 2006. PLoS ONE 3, e1549.
- Li A and Buchmann K (2001) Temperature- and salinity dependent development of a Nordic strain of Ichthyophthirius multifiliis. Journal of Applied Ichthyology 17, 273–276.
- Li X, He Y, Jaafar R, Kania PW and Buchmann K (2022) Effects of a Pseudomonas H6 surfactant on rainbow trout and Ichthyophthirius multifiliis: in vivo exposure. Aquaculture 547, 737479 (1–9).
- Lin D, Hua Y, Zhang Q, Xu D, Fu Y, Liu Y and Zhou S (2016) Evaluation of medicated feeds with antiparasitical and immune-enhanced Chinese herbal medicines against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idellus). Parasitology Research 115, 2473–2483.
- Lindenstrøm T and Buchmann K (2000) Acquired resistance in rainbow trout against Gyrodactylus derjavini. Journal of Helminthology 74, 155–160.
- Lyholt HCK and Buchmann K (1996) Diplostomum spathaceum: effects of temperature and light on cercarial shedding and infection of rainbow trout. Diseases of Aquatic Organisms 25, 169–173.
- Madsen HCK, Buchmann K and Mellergaard S (2000) Treatment of trichodiniasis in eel Anguilla anguilla in recirculated systems in Denmark: alternatives to formaldehyde. Aquaculture 186, 221–231.
- Mathiessen H, Jaafar R, Al-Jubury A, Jørgensen LVG, Kania PW and Buchmann K (2021a) Comparative in vitro and in vivo effects of feed additives on rainbow trout response to Ichthyophthirius multifiliis. North American Journal of Aquaculture 83, 1–11. doi:
- Mathiessen H, Marana MH, Korbut R, Wu B, Al-Jubury A, Karami AM, Kania PW and Buchmann K (2021b) Inflammatory reactions in rainbow trout fins and gills exposed to biocides. Diseases of Aquatic Organisms 146, 9–21.
- Meinelt TS, Matzke S, Stüber A, Pietrock M, Wienke A, Mitchell AJ and Straus DL (2009) Toxicity of peracetic acid (PAA) to tomonts of Ichthyophthirius multifiliis. Diseases of Aquatic Organisms 86, 51–56.
- Midtlyng P (2022) Current use and need for new fish vaccines. In Buchmann K and Secombes C (eds), Principles of Fish Immunology – From Cells and Molecules to Protection. Switzerland: Springer Nature. ISBN 978-3-030-854119-5.
- Mizuno S, Urawa S, Miyamoto M, Hatakeyama M, Sasaki Y, Koide NS and Ueda H (2018) Effects of dietary supplementation with oregano essential oil on prevention of the ectoparasitic protozoans Ichthyobodo salmonis and Trichodina truttae in juvenile chum salmon Oncorhynchus keta. Journal of Fish Biology 93, 528–539.
- Moen T, Torgersen J, Santi N, Davidson WS, Baranski M and Ødegård J (2015) Epithelial cadherin determines resistance to infectious pancreatic necrosis virus in Atlantic salmon. Genetics 200, 1313.
- Ogawa K and Yokoyama H (1998) Parasitic diseases of cultured marine fish in Japan. Fish Pathology 33, 303–309.
- O'Shea B, Wadsworth S, Pino-Marambio J, Birkett MA, Pickett JA and Mordue-Luntz AJ (2016) Disruption of host-seeking behaviour by the salmon louse Lepeophtheirus salmonis, using botanically derived repellants. Journal of Fish Diseases 40, 495–505. doi:
- Østevik L, Stormoen M, Evensen Ø, Xu C, Lie K-I, Nødtvedt A, Rodger H, Skagøy A, Manji F and Alarcon M (2022) Effects of thermal and mechanical delousing on gill health of farmed Atlantic salmon (Salmo salar L.). Aquaculture 552, 738019.
- Ozaki A, Yoshida K, Fuji K, Kubota S, Kai W and Aoki J (2013) Quantitative trait loci (QTL) associated with resistance to a monogenean parasite (Benedenia seriolae) in yellowtail (Seriola quinqueradiata) through genome wide analysis. PLoS ONE 8, e64987.
- Palenzuela O, Del Pozo R, Piazzon MC, Isern-Subich MM, Ceulemans S, Coutteau P and Sitjà-Bobadilla A (2020) Effect of a functional feed additive on mitigation of experimentally induced gilthead sea bream Sparus aurata enteromyxosis. Diseases of Aquatic Organisms 138, 111–120.
- Piazzon MC, Calduch-Giner JA, Fouz B, Estensoro I, Simo-Mirabet P, Puyalto M, Karalazos V, Palenzuela O, Sitja-Bobadilla A and Perez-Sanchez J (2017) Under control: how a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. BMC Microbiome 5, 18–23.
- Picon-Camacho SM, Marcos-Lopez M and Shinn AP (2012) An assessment of the use of drugs and non-drug intervention in the treatment of Ichthyophthirius multifiliis Fouquet, 1876, a protozoan parasite of freshwater fish. Parasitology 139, 149–190.
- Poley J, Purcell SL, Igboeli OO, Donkin A, Wotton H and Fast MD (2013) Combinatorial effects of administration immunostimulatory compounds in feed and follow-up administration of triple-dose SLICE® (emamectin benzoate) on Atlantic salmon Salmo salar L., infection with Lepeophtheirus salmonis. Journal of Fish Diseases 36, 299–309.
- Pool D, Ryder K and Andrews C (1984) The control of Bothriocephalus acheilognathi in grass carp, Ctenopharyngodon idella, using praziquantel. Fisheries Management 15, 31–33.
- Rach JJ, Gaikowski MP and Ramsay RT (2000) Efficacy of hydrogen peroxide to control parasitic infestation on hatchery-reared fish. Journal of Aquatic Animal Health 12, 267–273.
- Rigos G, Mladineo I, Nikoloudaki C, Vrbatovic A and Kogiannou D (2016) Application of compound mixture of caprylic acid, iron and mannan oligosaccharide against Sparicotyle chrysophrii (Monogenea: Polyopisthocotylea) in gilthead sea bream, Sparus aurata. Folia Parasitologica 63, 20–27.
- Rintamäki-Kinnunen P, Rahkonen M, Mannermaa-Keränen AL, Suomalainen LR, Mykrä H and Valtonen ET (2005) Treatment of ichthyophthiriasis after malachite green. I. Concrete tanks at salmonid farms. Diseases of Aquatic Organisms 64, 69–76.
- Robledo D, Matika O, Hamilton A and Houston RD (2018) Genome-wide association and genomic selection for resistance to amoebic gill disease in Atlantic salmon. G3 8, 1195–1203.
- Robledo D, Guitierrez AP, Barria A, Lhorente JP, Houston RD and Yanez JM (2019) Discovery and functional annotation of quantitative trait loci affecting resistance to sealice in Atlantic salmon. Frontiers in Genetics 10, 56.
- Rodriguez-Ramilo ST, Fernandez J, Toro MA, Bouza C and Hermida M (2013) Uncovering QTL for resistance and survival time Philasterides dicentrarchi in turbot (Scophthalmus maximus). Animal Genetics 44, 149–157.
- Sangmaneedet S and Smith SA (1999) Efficacy of various chemotherapeutic agents on the growth of Spironucleus vortens, an intestinal parasite of the freshwater angelfish. Diseases of Aquatic Organisms 38, 47–52.
- Schmahl G and Mehlhorn H (1985) Treatment of fish parasites I. Praziquantel effective against monogenea (Dactylogyrus vastator, Dactylogyrus extensus, Diplozoon paradoxum). Zeitschrift für Parasitenkunde 71, 727–737.
- Schmahl G, Mehlhorn H and Tarascewski H (1989) Treatment of fish parasites: 5. The effects of sym. triazinone (Toltrazuril) on fish parasitic ciliophora (Ichthyophthirius multifiliis Fouquet, 1876, Apiosoma amoebea Grenfell, 1884, Trichodina sp. Ehrenberg, 1831). European Journal of Protistology 24, 52–161.
- Shao C, Niu Y, Rastas P, Liu Y, Xie Z, Li H, Wang L, Jiang Y, Tai S, Tian Y, Sakamoto T and Chen S (2015) Genome-wide SNP identification for the construction of a high-resolution genetic map of Japanese flounder (Paralichthys olivaceus): applications to QTL mapping of Vibrio anguillarum disease resistance and comparative genomic analysis. DNA Research 22, 161–170.
- Shinn A, Picon-Camacho S, Bawden R and Taylor N (2009) Mechanical control of Ichthyophthirius multifiliis Fouquet, 1876 (Ciliophora) in a rainbow trout hatchery. Aquacultural Engineering 41, 152–157.
- Shinn AP, Pratoomyot J, Bron JE, Paladini G, Brooker EE and Brooker AJ (2015) Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 142, 196–270.
- Sigh J and Buchmann K (2001) Comparison of immobilization assays and enzyme-linked immunosorbent assays for detection of rainbow trout antibody-titres against Ichthyophthirius multifiliis Fouquet, 1876. Journal of Fish Diseases 24, 49–51.
- Sitjà-Bobadilla A, Felipe MC and Alvarez-Pellitero P (2006) In vivo and in vitro treatments against Sparicotyle chrysophrii (Monogenea: Microcotylidae) parasitizing the gills of gilthead seabream (Sparus aurata L.). Aquaculture 261, 856–864.
- Sommerville C, Endris R, Bell TA, Ogawa K, Buchmann K and Sweeney D (2016) World association for the advancement of veterinary parasitology (WAAVP) guideline for testing the efficacy of ectoparasiticides for fish. Veterinary Parasitology 219, 84–99.
- Stone J, Roy WJ, Sutherland IH, Ferguson HW, Sommerville C and Endris R (2002) Safety and efficacy of emamectin benzoate administered in-feed to Atlantic salmon, Salmo salar L., smolts in freshwater as a preventive treatment against infestations of sea lice, Lepeophtheirus salmonis (Kroyer). Aquaculture 210, 21–34.
- Straus D and Griffin B (2001) Prevention of an initial infestation of Ichthyophthirius multifiliis in channel catfish and blue tilapia by potassium permanganate treatment. North American Journal of Aquaculture 63, 11–16.
- Straus DL and Meinelt T (2009) Acute toxicity of peracetic acid (PAA) formulations to Ichthyophthirius multifiliis theronts. Parasitology Research 104, 1237–1241.
- Szekely C and Molnar K (1987) Mebendazole is an efficacious drug against pseudodactylogyrosis in the European eel (Anguilla anguilla). Journal of Applied Ichthyology 3, 183–186.
- Szekely C, Molnar K and Baska F (1988) Efficacy of fumagillin against Myxidium giardi Cepede, 1906 infections of the European eel (Anguilla anguilla): new observations on myxidiosis of imported glass-eels. Acta Veterinaria Hungarica 36, 239–246.
- Tedesco P, Beraldo P, Massimo M, Fioravanti ML, Volpatti D, Dirks R and Galuppi R (2020) Comparative therapeutic effects of natural compounds against Saprolegnia spp. (Oomycota) and Amyloodinium ocellatum (Dinophyceae). Frontiers in Veterinary Science 7, 83.
- Tokşen E and Nemli E (2010) Oral treatment trials on telescope fish (Carassius auratus) experimentally infected with Ichthyophthirius multifiliis (Fouquet, 1876). Bulletin of the European Association for Fish Pathologists 30, 48–54.
- Vallejo RL, Palti Y, Liu S, Evenhuis JP, Gao G, Rexroad III CE and Wiens GD (2014) Detection of QTL in rainbow trout affecting survival when challenged with Flavobacterium psychrophilum. Marine Biotechnology 16, 349–360. doi.
- Verrier ER, Dorson M, Mauger S, Torhy C, Ciobotaru C, Hervet C, Dechamp N, Genet C, Boudinot P and Quillet E (2013) Resistance to rhabdovirus (VHSV) in rainbow trout: identification of a major QTL related to innate mechanisms. PLoS ONE 8, e55302.
- Waller PJ and Buchmann K (2001) Anthelmintic resistance and parasite control in commercial eel farms: consequences for producers. Veterinary Record 148, 783–784.
- Wang L, Fan C, Liu Y, Zhang Y, Liu S, Sun D, Deng H, Xu Y, Tian Y, Liao X, Xie M, Li W and Chen S (2014) A genome scan for quantitative trait loci associated with Vibrio anguillarum infection resistance in Japanese Flounder (Paralichthys olivaceus) by bulked segregant analysis. Marine Biotechnology 16, 513–521.
- Wiens G, Vallejo RL, Leeds TD, Palti Y, Hadidi SS and Liu S (2013) Genetic correlation between cold water disease resistance and spleen index in a domesticated population of rainbow trout: identification of QTL on chromosome Omy19. PLoS ONE 8, e75749.
- Woo PTK (1981) Acquired immunity against Trypanosoma danilewski in goldfish Carassius auratus. Parasitology 83, 343–346.
- Woo PTK and Shariff M (1990) Lernaea cyprinacea L. (Copepoda: Caligidae) in Helostoma temmincki Cuvier & Valencienne: the dynamics of resistance in recovered and naive fish. Journal of Fish Diseases 13, 485–493.
- Woo PTK, Leong AL and Buchmann K (2020) Climate change and infectious fish diseases (CCIFD) sections I, II, III. CABI, UK. 514 pp. CAB International Oxon, UK. ISBN 978-1789243277.
- Xueqin J, Kania PW and Buchmann K (2012) Comparative effects of four feed types on white spot disease susceptibility and skin immune parameters in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases 35, 127–135.
- Zheng ZL, Tan JYW, Liu HY, Zhou XH and Wang KY (2009) Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 292, 214–218.
