Introduction
Ketamine has emerged as a rapid-acting antidepressant, with the S-enantiomer of the drug (esketamine) receiving US Food and Drug Administration approval for treatment-resistant depression (TRD) in 2019 []. While ongoing treatment can prevent relapse [], concerns exist regarding the feasibility of long-term treatment. Currently, the time and financial costs to patients can be extensive, with a mandatory 2-h observation period, driving restrictions on days of treatment, and continued treatment weekly or every other week indefinitely for maintenance of effect [-]. These restrictions cause significant disruption to patients’ lives and can potentially limit the patient flow at treatment centers. Furthermore, while long-term safety data with esketamine suggests that treatment for up to 1 year at antidepressant doses are safe [], concerns continue to be expressed that long-term ketamine/esketamine exposure may increase abuse liability and lead to other potential adverse outcomes []. Treatment approaches that reduce or eliminate the need for ongoing ketamine/esketamine maintenance treatments are needed.
Murrough et al. [] suggested that long-term solutions should be “guided by hypothesized mechanistic synergy.” Traditionally, it was thought that NMDA receptor antagonism mediated the drug’s therapeutic effect []. More recently, other mechanisms have been proposed [, ]. Regardless of the proximate neurobiological mechanism, there is strong evidence that an enhancement in synaptic neuroplasticity is critical for ketamine’s antidepressant effect []. The enhanced levels of neuroplasticity induced by ketamine have been associated with the drug’s ability to enhance and reverse impaired levels of cognitive flexibility that may be a feature underlying depressed individuals’ inability to appropriately adapt to environmental changes [-]. We have hypothesized that ketamine may provide an opportune window during which cognitive and behavioral interventions may be used to harness a state of enhanced neuroplasticity [, ]. Cognitive behavioral therapy (CBT) is highly effective in relapse prevention, especially among those with previous recurrent episodes [, ]. Ketamine, with its rapid-acting and neuroplasticity-enhancing effects, may in turn help patients by improving early engagement in CBT through alleviation of symptoms and facilitating the neurobiological mechanisms of learning. This treatment combination of ketamine in conjunction with CBT may lead to improved longer-term outcomes while minimizing the need for ongoing exposure to ketamine []. Building on prior work [], we conducted a randomized, proof-of-concept trial to examine the efficacy of CBT in sustaining the antidepressant effects of ketamine in patients with TRD. Following this earlier work, this approach is based on the sequential treatment model [, ]. We hypothesized that CBT would lead to improved longer-term outcomes following ketamine.
Methods
Subjects and Eligibility
Subjects with severe major depressive disorder were recruited from among patients seeking clinical treatment with ketamine at the Yale Interventional Psychiatric Service from February 2017 through August 2019. The inclusion criteria included: (1) treatment resistance to 2 or more adequate courses of antidepressant medications (as measured by the MGH-Antidepressant Treatment History Questionnaire []), (2) a severe depressive episode as measured by a score of 21 or greater on the 17-Item Hamilton Depression Rating Scale [], and (3) age between 18 and 65. Subjects with active substance use (other than nicotine) and/or comorbid schizophrenia/schizoaffective disorder were excluded.
Protocol
Subjects were treated with 6 intravenous infusions of ketamine (0.5 mg/kg over 40 min) over 3 weeks, which comprised phase 1. Following these 6 ketamine infusions, subjects who experienced a clinical response (≥50% improvement in depression severity by the end of phase 1) were randomly assigned to receiving CBT or treatment as usual (TAU) for an additional 14 weeks (phase 2; Fig. 1). Randomization was conducted via a unique computer-generated randomization code (1:1 ratio) in randomly varying blocks of 2–4. Randomization was carried out by a biostatistician with no hands-on involvement in the trial. Depression severity over time was measured using the Montgomery-Asberg Depression Rating Scale (MADRS) (primary outcome measure) [] and the Quick Inventory of Depressive Symptomatology, Self-Report 16-Item (QIDS-SR-16) []. The MADRS was conducted remotely via telephone by raters who were blind to treatment allocation. Prior research suggests a comparable validity of the MADRS conducted in-person and by phone []. Following the completion of phase 1, patients did not receive additional ketamine infusions during phase 2.
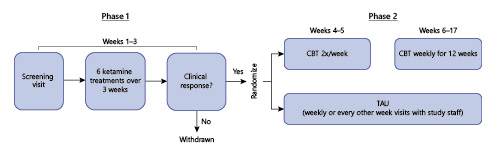
Fig. 1
Trial schema.
Cognitive Behavioral Therapy
CBT was based on the Beck model and was comprised of: (1) psychoeducation, (2) cognitive restructuring, and (3) behavioral activation. CBT was conducted by 2 experienced therapist (M.K.F. and L.F.) who had received prior training and certification from the Beck Institute for CBT and had participated in several prior clinical trials involving CBT [, , ]. Patients received twice weekly CBT sessions during the first 2 weeks of phase 2 and weekly sessions thereafter. Homework included “activity charts” and “thought records” to assist patients in the internalization and adoption of CBT principles. Each session began with an agenda and included follow-up of homework from the prior session and subsequent homework assignments.
Treatment as Usual
TAU consisted of weekly or every-other-week visits with a study physician. The focus of these visits was medication management and management of adverse events. Oral antidepressant medications could be adjusted by the clinician based on the clinical presentation.
Cognitive Assessments
During the study, an amendment to the protocol was made to add a neuropsychological assessment before and after ketamine (phase 1). This consisted of 2 tasks intended to assess cognitive control and flexibility. An emotional N-back task (2-back version; neutral, positive, and negative words) was used to assess cognitive control. This task assesses multiple components of executive control (including updating and maintenance) and has demonstrated acceptable construct reliability and validity []. Participants are asked to observe single words presented on a screen and to decide whether the currently presented word is the same or different from the one presented 2 words back. Accuracy is the outcome measure for this task. For positive and negative trials, words were selected from the Affective Norms of English Words List []. The emotional Stroop was used to assess cognitive control, specifically interference inhibition. A colored word stimulus is presented and participants are instructed to indicate as quickly as possible the color in which the presented word is written and to ignore the meaning of the word. The stimulus words appear randomly in 1 of 3 presentation colors, i.e., blue, green, and yellow. In this emotional Stroop, the words used have positive, negative, neutral, or threat connotations according to the Affective Norms of English Words List []. The reaction times to the words indicate the degree of interference. In previous research, scores on the emotional Stroop task have been found to be stable over a 6-month period for people who remain depressed [].
Analysis
For baseline demographic and clinical characteristics, groups were compared on continuous variables using two-sample t tests and on categorical measures using the Pearson χ2 test. QIDS and MADRS forms were collected each week from all of the study participants, and we calculated summary scores by week for all of the participants [, , ]. We normalized the scores by subtracting the baseline scores (week 1) from each subsequent week’s scores to indicate the severity of depressive symptoms. A more negative value indicates improvement in depressive symptoms. We presented improvement in depressive symptoms by group and week using QIDS and MADRS scales. To test for a time-by-treatment group interaction, we used analysis of variance, controlling for repeated measures. All analyses were conducted in STATA 15.1 MP/6-Core (Stata Corp., College Station, Texas). This study is registered in the Clinical Trials Registry (NCT03027362).
Results
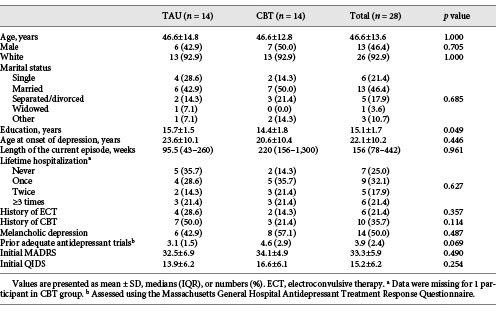
Clinical and Sociodemographic Characteristics
Forty-two patients signed informed consent, with 41 entering phase 1 and undergoing 1 or more ketamine infusions (Fig. 2). Of these, 28 patients were eligible for and underwent randomization to CBT or TAU alone in phase 2. Of these 28 patients, the majority were female (53.6%), white (92.9%), and well educated (mean years of education: 15.1; SD = 1.7). These patients were chronically and severely ill; 20 out of 28 (71%) reported 1 or more prior hospitalizations, 6 out of 28 (21%) had received electroconvulsive therapy, and 14 out of 28 (50%) had a melancholic subtype of depression. The sample had a median duration of the current episode of depression of 156 weeks (Table 1).
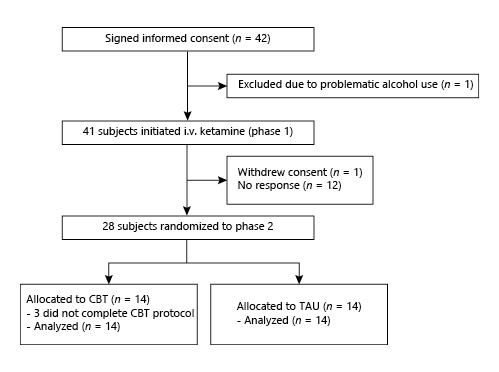
Fig. 2
Online supplementary CONSORT diagram (for all online suppl. material, see http://www.karger.com/doi/10.1150/000517074).
Depression Outcomes
When measured by the Montgomery-Asberg Depression Rating Scale (primary outcome measure), the effect size was moderate (Cohen’s d = 0.65; 95% CI –0.55 to 1.82) at the end of the study; the time-by-treatment interaction was not significant, however. There was a significant interaction effect of time and treatment group as measured by the Quick Inventory of Depressive Symptomatology (F = 4.58; p = 0.033), favoring a greater sustained improvement in the CBT group (Fig. 3). This corresponded to a moderate-to-large effect size of the Cohen (d = 0.71; 95% CI –0.30 to 1.70) at the end of the study (14 weeks following the last ketamine infusion).
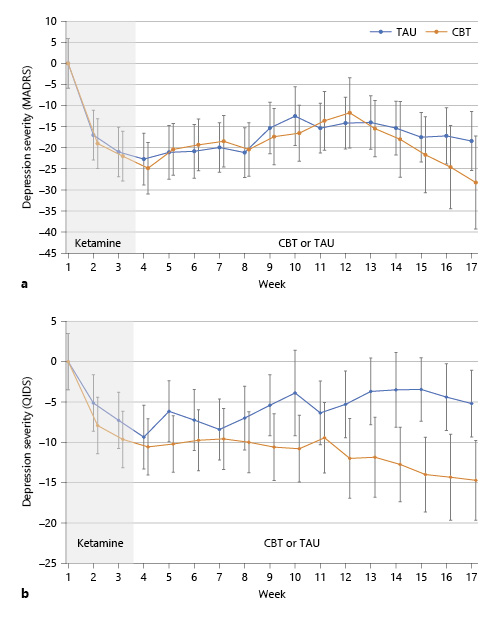
Fig. 3
Depressive symptoms by time and treatment group. Patients underwent intravenous ketamine for 3 weeks and then randomization to CBT or TAU. a Interaction of treatment and time was not statistically significant as measured by the MADRS. b The interaction of treatment group and time was statistically significant (F = 4.58; p = 0.033) as measured by the QIDS. Error bars represent standard error of the mean.
Measures of Cognitive Control
A subset of patients (N = 9 responders; N = 11 nonresponders) underwent cognitive testing using the emotional Stroop and N-back assessments before and after ketamine (Fig. 4). In these measures of cognitive flexibility, patients who responded to ketamine showed improvement in the accuracy of emotional N-back; (t[8] = 2.33; p < 0.05) whereas nonresponders did not (t[10] <1; ns). Responders also showed greater improvement in reaction time in the valence emotional Stroop task compared to nonresponders, though this was not statistically significant.
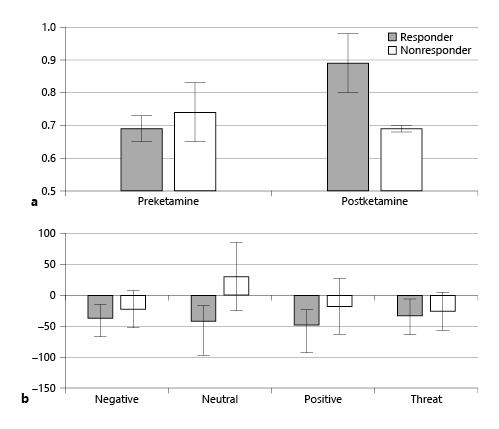
Fig. 4
Cognitive control measures before and after ketamine in responders vs. nonresponders. a Patients who experience a response to ketamine show an improvement in the accuracy of emotional N-back following treatment, whereas nonresponders do not. b Ketamine responders show greater improvement in reaction time (ms) on the emotional Stroop tasks compared to nonresponders. A lower score indicates more improvement. For both panels: total N = 20; responders = 9; nonresponders = 11.
Safety
The protocol was generally well tolerated. There were no treatment-emergent adverse events attributable to the protocol that led to discontinuation. There were 5 serious adverse events, i.e., 3 in the CBT group and 2 in the TAU group. All involved a worsening of depression or suicidal ideation and the need for hospitalization; there was 1 aborted suicide attempt (CBT group) and 1 suicidal gesture (TAU group; a woman wrote a suicide note and drank a small amount of alcohol while in the bathtub). None of these events were considered related to the treatment protocol but were thought to be related to relapse/worsening of depression after some time following ketamine. The most common adverse events were headache (occurring in 24.4% [10 out of 41] of the patients, generally mild in nature, and judged related to ketamine exposure) and nausea (occurring in 12.2% [5 out of 41] of the patients, mild in nature, and judged related to ketamine exposure). Transient fatigue occurred in 2 patients and was considered possibly or likely related to ketamine. Other adverse events occurred in only 1 patient each and (with the exception of transient lip numbness) were judged not related to ketamine (constipation, upper respiratory tract infection, worsening insomnia, myalgia, jaw/mouth soreness, pruritus, noncardiac chest pain, and neuropathy exacerbation). No adverse events were judged attributable to CBT.
Discussion
This study provides preliminary data that CBT may sustain the antidepressant effects of intravenous ketamine in patients with TRD. In this pilot randomized trial, CBT treatment suggested a moderate effect (d = 0.65) on the clinician-administered MADRS (a priori primary outcome measure) compared to TAU at the end of the study, though the time-by-treatment interaction was not significant. When measured by a self-reported outcome (QIDS), there was a statistically significant time-by-treatment interaction favoring CBT. In a subset of patients, changes in cognitive control processes (i.e., emotional Stroop and N-back) were associated with clinical improvement following ketamine treatment.
There are at least 2 mechanisms whereby the combination of ketamine and CBT may lead to improved longer-term outcomes. First, it is possible that both treatments produce similar effects, but with differing time courses. Along with its rapid antidepressant properties, ketamine may rapidly improve cognitive control [-]; CBT may then strengthen and maintain these improvements in cognitive control []. It has long been hypothesized that CBT produces its antidepressant and prophylactic effects by attenuating and reversing inaccurate beliefs and the maladaptive information processing which form the basis for repetitive negative thinking and may have a causal role in depression. Thus, when maladaptive thinking is corrected, acute distress and the future risk of relapse will both be reduced. Several groups have suggested that these changes in maladaptive thinking are mediated through effects on cognitive control processes such as inhibition/suppression, flexible updating, active maintenance, and interference control [, ]. Interestingly, recent work also suggests that ketamine may produce similar effects on cognitive control processes within a short period of time following administration. Preclinical work in rodents suggests that sensitivity to negative stimuli and distraction by negative feedback are decreased after ketamine treatment in a probabilistic reversal learning task []. Other studies have demonstrated the ability of ketamine to rapidly correct for stress-induced decreases in reversal learning [, ] and improve flexible control in processing affective material.
The second mechanism by which ketamine and CBT may lead to improved outcomes is related to the hypothesis that ketamine can induce a state of enhanced neuroplasticity in critical brain regions. This state may facilitate and enhance the ability of CBT to produce long-lasting changes in the brain, resulting in improved cognitive control processing, reduced depressive symptomology, and prevention of relapse. In other words, ketamine exposure may increase the general sensitivity of synapses within key brain regions (i.e., medial prefrontal cortex and hippocampus) to plasticity-inducing events, such as is proposed through the use of CBT []. There is mounting evidence that ketamine administration is associated with a rapid onset of structural and functional changes within the brain. At the molecular level, ketamine has been shown to have complex metaplastic effects on hippocampal slice preparations []. Ketamine has also been shown to reverse stress-induced deficits in reversal learning [], enhance extinction of fear conditioning [], and enhance hippocampal long-term potentiation and facilitate extinction of avoidance behavior in drug-responsive Wistar-Kyoto rats (an animal model of endogenous depression []), with effects lasting at least 3 weeks []. Moreover, ketamine has been shown to have a rapid onset of marked effects on dendritic architecture and spine density and function in prefrontal cortical regions that persist for at least 1 week after a single administration [] and may be extended with subchronic dosing []. Further, there is preliminary evidence that ketamine can improve negative self-schema, which is one of the key psychotherapeutic targets of CBT []. Although several important mechanistic details remain to be elucidated, there is increasing evidence from our group and others suggesting overall improvement in gross cognitive function following a series of ketamine treatments [, -]. Based on these data we hypothesize that there will be a period of increased flexible learning associated with ketamine treatments and that this period of increased learning potential will allow for an enhanced ability of CBT to improve longer-term outcome.
The data from this proof-of-concept study cannot definitively support one hypothesis more than another. It is recommended that future, well-powered trials be funded to: (1) confirm that the combination of ketamine and CBT lead to improved longer-term outcomes compared to ketamine alone and (2) test mechanisms through which these improved outcomes may be mediated. Further questions about the timing of CBT delivery with respect to ketamine are also worthy of investigation. While the study showed a significant time-by-treatment interaction using the QIDS, this significance was not achieved using the MADRS assessment. The small sample size of the study might explain this discrepancy, although another possible reason is that the MADRS assessment was performed by blinded raters using phone interviews, whereas the QIDS is a self-report where blinding was not possible given the nature of the treatments. It is also possible that the QIDS and the MADRS capture slightly different symptom profiles, which could be part of the reason for these discrepant findings.
The treatment protocol was generally well tolerated, with no treatment-emergent adverse events that led to discontinuation. The most prevalent adverse events related to ketamine (i.e., transient nausea and headache) were expected and consistent with other ketamine clinical trials [-]. Notably, there were a number of serious adverse events which were related to relapse/worsening of depression/suicidal ideation. These adverse events were generally considered relapses within the context of the natural course of major depressive disorder and are likely a reflection of either the illness severity of the sample (Table 1) or the well-characterized, transient antidepressant benefits associated with a time-limited course of ketamine administration [, ]. However, it has been suggested that ketamine (or one of its enantiomers) may have a withdrawal effect and may lead to adverse events following discontinuation []. Hence, although there was no evidence of a classic withdrawal syndrome associated with ketamine as provided in this protocol, it is possible that this rapid return of depressive symptoms and suicidal ideation following discontinuation of the drug may constitute a form of withdrawal, as noted by Cosci and Chouinard []. Given that this protocol had a time-limited exposure period for ketamine, future trials may consider a tapered approach to discontinuation to mitigate a potential relapse associated with abrupt discontinuation or a withdrawal side effect. Regardless, investigators and clinicians must be extremely cautious in administration of ketamine to severely ill patients. The implementation of a strict Risk Evaluation and Mitigation Strategy (REMS) in concert with the FDA approval of esketamine is hoped to minimize the risk associated with this drug [].
Several limitations require comment. First, this was an open-label investigation with respect to ketamine and no subject received placebo as a lead-in to CBT versus TAU randomization. Second, due to funding constraints and the pilot nature of this study, the sample size was small. Third, due to the nature of the treatment, it was not possible to blind participants to the study group allocation. Fourth, the MADRS ratings were conducted by phone to help preserve the blinding of the raters; future studies might incorporate in-person or remote means for ratings that provide visual as well as audio communication between the raters and subjects. Finally, given that most patients were chronically and severely depressed, medications were not strictly controlled.
Conclusions
This pilot study provides preliminary data that CBT may sustain the antidepressant effects of ketamine in TRD when used in a sequential model approach. While they are admittedly speculative at this point, these treatments may combine synergistically; ketamine may enhance metaplasticity and CBT may stabilize synaptic connections to improve long-term outcomes [, , ]. An alternative hypothesis is that ketamine may resolve depression-related cognitive distortions and CBT may act to sustain these resolutions and maintain therapeutic effects. Given this promising pilot data, further study and optimization of this treatment approach in well-powered clinical trials is recommended.
Statement of Ethics
Written informed consent was obtained from all of the participants. This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board at Yale School of Medicine (HIC No. 1609018450).
Conflict of Interest Statement
Dr. Wilkinson has received contract funding from Janssen, Sage Therapeutics, and Oui Therapeutics for the conduction of clinical trials administered through Yale University; he has received consulting fees from Biohaven Pharmaceuticals, Sage Therapeutics, Janssen, and Oui Therapeutics. Dr. Kitay has received contract funding from Janssen, Sage Therapeutics, and for the conduction of clinical trials administered through Yale University; he has received consulting fees from Janssen and Otsuka Pharmaceutical. Dr. Sanacora has received consulting fees from Allergan, Alkermes, AstraZeneca, Avanier, Axsome Therapeutics, Pharmaceuticals, Biohaven Pharmaceuticals, Bristol-Myers Squibb, Clexio Biosciences, EMA Wellness, Epiodyne, Intra-Cellular Therapies, Janssen, Merck & Co., Naurex, Navitor, NeruoRx, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis Therapeutics, Sage Pharmaceuticals, Servier Pharmaceuticals, Taisho Pharmaceuticals, Teva, Valeant, and Vistagen therapeutics over the last 36 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Hoffman La-Roche, Merck, Naurex, and Servier over the last 36 months. Free medication was provided to GS for an NIH-sponsored study by Sanofi-Aventis. In addition, he holds shares in BioHaven Pharmaceuticals Holding Company and is a coinventor on a patent “Glutamate agents in the treatment of mental disorders” (patent No. 8778979), and a U.S. Provisional Patent Application (No. 047162-7177P1; 00754) filed on August 20, 2018, by the Yale University Office of Cooperative Research (OCR 7451 US01).
The other authors report no financial relationships with commercial interests.
Funding Sources
This work was funded by a young investigator grant from the Brain and Behavior Research Foundation (formerly NARSAD) and the Robert E. Leet and Clara Gurthrie Patterson Trust (S.T.W.). It was also supported by the Agency for Healthcare Research and Quality (K12HS023000; S.T.W.).
Author Contributions
Substantial contributions to the conception or design of this work: S.T.W. and G.S. Acquisition, analysis, and interpretation of data for this work: all of the authors. Drafting of this work or critical revision for important intellectual content: all of the authors. Final approval of the version to be published: all of the authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all of the authors.
References
- 1. Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381(1):1–4.
- 2. Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893–903.
- 3. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039–40.
- 4.
- 5. Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, et al Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 2020;81(3):e1–10.
- 6. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422–4.
- 7. Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6.
- 8. Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64.
- 9. Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6.
- 10. Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205–15.
- 11. Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology (Berl). 2015;232(17):3123–33.
- 12. Paredes D, Silva JD, Morilak DA. Ketamine corrects a deficit in reversal learning caused by chronic intermittent cold stress in female rats. Int J Neuropsychopharmacol. 2018;21(12):1109–13.
- 13. Patton MS, Lodge DJ, Morilak DA, Girotti M. Ketamine corrects stress-induced cognitive dysfunction through JAK2/STAT3 signaling in the orbitofrontal cortex. Neuropsychopharmacology. 2017;42(6):1220–30.
- 14. Uddin LQ. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat Rev Neurosci. 2021;22(3):167–79.
- 15. Wilkinson ST, Wright D, Fasula MK, Fenton L, Griepp M, Ostroff RB, et al Cognitive Behavior Therapy may sustain antidepressant effects of intravenous ketamine in treatment-resistant depression. Psychother Psychosom. 2017;86(3):162–7.
- 16. Wilkinson ST, Holtzheimer PE, Gao S, Kirwin DS, Price RB. Leveraging neuroplasticity to enhance adaptive learning: the potential for synergistic somatic-behavioral treatment combinations to improve clinical outcomes in depression. Biol Psychiatry. 2019;85(6):454–65.
- 17. Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417–22.
- 18. Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;19(2):pyv076.
- 19. Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. 2016;173(2):128–37.
- 20. Guidi J, Fava GA. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):261–9.
- 21. Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322–5.
- 22. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62.
- 23. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–9.
- 24. Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83.
- 25. Kobak KA, Williams JB, Jeglic E, Salvucci D, Sharp IR. Face-to-face versus remote administration of the Montgomery-Asberg Depression Rating Scale using videoconference and telephone. Depress Anxiety. 2008;25(11):913–9.
- 26. Sanacora G, Fenton LR, Fasula MK, Rothman DL, Levin Y, Krystal JH, et al Cortical gamma-aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry. 2006;59(3):284–6.
- 27. Fenton L, Fasula M, Ostroff R, Sanacora G. Can cognitive behavioral therapy reduce relapse rates of depression after ECT? a preliminary study. J ECT. 2006;22(3):196–8.
- 28. Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137(2):201–25.
- 29. Bradley MM, Lang PJ. Affective norms for English words (ANEW): instruction manual and affective ratings. Technical Report C-1, The Center for Research in Psychophysiology, University of Florida; 1999.
- 30. Dozois DJ, Dobson KS. A longitudinal investigation of information processing and cognitive organization in clinical depression: stability of schematic interconnectedness. J Consult Clin Psychol. 2001;69(6):914–25.
- 31. Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Asberg Depression Scale: reliability and validity. Acta Psychiatr Scand. 1986;73(5):544–8.
- 32. Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82.
- 33. Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, et al Cognitive Behavioral Therapy is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(4):311–9.
- 34. Joormann J, Vanderlind WM. Emotion regulation in depression: the role of biased cognition and reduced cognitive control. Clin Psychol Sci. 2014;2(4):402–21.
- 35. Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63(4):377–84.
- 36. Rychlik M, Bollen E, Rygula R. Ketamine decreases sensitivity of male rats to misleading negative feedback in a probabilistic reversal-learning task. Psychopharmacology (Berl). 2017;234(4):613–20.
- 37. Izumi Y, Zorumski CF. Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacology. 2014;86:273–81.
- 38. Girgenti MJ, Ghosal S, LoPresto D, Taylor JR, Duman RS. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol Dis. 2017;100:1–8.
- 39. Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8(11):925–32.
- 40. Fortress AM, Smith IM, Pang KC. Ketamine facilitates extinction of avoidance behavior and enhances synaptic plasticity in a rat model of anxiety vulnerability: implications for the pathophysiology and treatment of anxiety disorders. Neuropharmacology. 2018;137:372–81.
- 41. Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49.
- 42. Pryazhnikov E, Mugantseva E, Casarotto P, Kolikova J, Fred SM, Toptunov D, et al Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine. Sci Rep. 2018;8(1):6464.
- 43. Hasler G, Suker S, Schoretsanitis G, Mihov Y. Sustained improvement of negative self-schema after a single ketamine infusion: an open-label study. Front Neurosci. 2020;14:687.
- 44. Wilkinson ST, Katz RB, Toprak M, Webler R, Ostroff RB, Sanacora G. Acute and longer-term outcomes using ketamine as a clinical treatment at the Yale Psychiatric Hospital. J Clin Psychiatry. 2018;79(4):e1–7.
- 45. Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, et al Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol. 2014;28(6):536–44.
- 46. Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17(11):1805–13.
- 47. Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64.
- 48. Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46.
- 49. Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42.
- 50. Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, et al Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123–9.
- 51. Cosci F, Chouinard G. Acute and persistent withdrawal syndromes following discontinuation of psychotropic medications. Psychother Psychosom. 2020;89(5):283–306.
- 52. Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25(3):530–43.
- 53. Hasler G. Toward specific ways to combine ketamine and psychotherapy in treating depression. CNS Spectr. 2020;25(3):445–7.