Background
Acute respiratory distress syndrome (ARDS) is a high-mortality disease with limited long-term quality of life eliciting widespread concern. Mechanical ventilation has been widely used as supportive therapy in ARDS patients for improving survival. Increasing evidence shows that optimal lung-protective mechanical ventilation, including low tidal ventilation, positive end-expiratory pressure (PEEP), and lung recruitment maneuvers (LRMs), may improve prognosis [-]. The purpose of these strategies is to minimize the size of unavailable lung area, amplify stress in alveolar units, and avoid atelectasis or overextension [, ]. Some research indicates that all strategies could be a double-edged sword, inappropriate ventilation may result in ventilator-induced barotrauma and initiate a series of severe inflammatory activities [, ]. Recruitment maneuver, considered the most controversial among all, has been widely studied. Despite the existence of a variety of recruitment maneuvers, it is poorly understood which one is the most effective as the low research quality. LRMs were defined as maneuvers assisting transient elevation of the driving pressure to recruit collapsed alveoli, but maximal lung recruitment could cause overextension of the lung [], whereas minimal recruitment could be ineffective. In two previous systematic reviews, Goligher et al. [] and Hodgson et al. [] demonstrated that LRMs reduced mortality in patients with ARDS, but the results lacked sufficient credibility because of the considerable number of co-interventions and lack of multicenter research data in the last 2 years. Furthermore, another recent study showed that routine use of LRM and PEEP titration was not supported in ARDS patients []. Therefore, the aforementioned evidence revealed that there was no consensus on whether LRMs should be used in ARDS patients, and its benefits remain undetermined.
Thus, we conducted a systematic review and meta-analysis that focused on determining whether LRMs could shorten the 28-day and in-hospital mortality, reduce the length of intensive care unit (ICU) and hospital stay, improve arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2) ratio, and decrease oxygen requirement in randomized controlled trials (RCTs) in ARDS patients.
Methods
In meta-analysis, patient consent is not a requirement, and ethical approval can be waived as well. This meta-analysis has been registered on https://www.crd.york.ac.uk/prospero/ with the registration number CRD42018102834.
Literature Review and Search Strategy
According to the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions statement, as well as Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) guidelines, PubMed, EMBASE, Ovid Medline, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched without language restrictions up to May 2018. Furthermore, information from gray literature was captured by searching the first 10 pages of Google scholar.
Various combinations, keywords and MeSH terms relevant to ARDS were used to perform the search. The terms used included “ARDS” or “acute chest syndrome” or “respiratory distress syndrome” or “shock lung” or “adult respiratory distress syndrome” or “respiratory distress syndrome, acute” or “human ARDS” or “ARDSs, human.” There were no MeSH terms relevant to LRMs, hence, the search items used were “lung recruitment maneuver” or “recruitment manoeuver” or “lung volume recruitment” or “lung recruitment” or “recruit manoeur” or “recruit manouev” or “recruit maneuv” or “recruit manuev” on the basis of a previous article []. Then, we combined the above results with the RCT’s MeSH or its related words to obtain the results.
Criteria for Considering Studies for this Review
Types of Studies
We retrieved RCTs that met the inclusion criteria. The cross-trials were excluded because they were not suitable for this review.
Types of Participants
Inclusion criteria were: (1) population: adults (≥18 years), diagnosed with ARDS, and undergoing mechanical ventilation; (2) intervention: using LRM technique defined as application of transient elevations to airway pressure during mechanical ventilation to open (“recruit”) collapsed lung units and increase the number of alveoli participating in tidal ventilation, including staircase or continuous positive airway pressure; (3) comparison: non-LRMs; (4) outcomes: refer to “Type of Outcome Measures”; (5) design: RCTs.
Criteria for exclusion were any nonhuman research.
Type of Outcome Measures
Based on the searched results, we selected the primary outcomes to be: 28-day and in-hospital mortality. The secondary outcomes were the length of ICU stay, the length of hospital stay, PaO2/FiO2, and FiO2. In-hospital mortality was considered as 60-day mortality if in-hospital mortality was unreported. The PaO2/FiO2 and FiO2were defined as data on the third day (2- to 7-day data were chosen in that order of preference if the third-day data were unreported). At least one of the primary outcomes was reported by qualified articles.
Data Selection
Two investigators (Yu Cui, Rong Cao) sequentially reviewed all titles, abstracts, and the full texts. They then determined enrolled trials and retrieved eligibility, quality, and outcomes independently. Disagreements over eligibility between the two reviewers were resolved by discussion. If necessary, the third reviewer (Yu Wang) was involved and adjudicated. Duplicate reports, non-RCTs, case reports, reviews, pediatric, and nonhuman experimental designs were eliminated. Additionally, conference abstracts and study protocols were also excluded unless published as full-text reports in journals.
The relevant data were collected by Yu Cui, Rong Cao, and Yu Wang as follows: first author, year of publication, study design, sample size, intervention description, control description, 28-day mortality, in-hospital mortality, length of ICU stay, and length of hospital stay, as well as PaO2/FiO2 and FiO2 on the third day. The first reviewer (Yu Cui) entered the data, and the others (Rong Cao, Yu Wang) double-checked for data accuracy.
Quality Assessment
Cochrane collaboration risk of bias, a classical and widely used quality assessment tool, was utilized for quality assessment. This included random sequence generation, allocation concealment, performance bias, detection bias, attribution bias, reporting bias, and others. According to the instruction, the risk of bias was judged at three levels (low risk of bias, unclear risk of bias and high risk of bias) from the aforementioned seven parts based on the original contents. Risk of bias analysis was performed by Review Manager® Version 5.3 for Windows (RevMan, The Cochrane Collaboration, Oxford, UK) and GRADEpro (McMaster University, Hamilton, ON, Canada, 2014).
Statistical Analysis
The variables were expressed as means ± standard deviations (SD) for continuous variables and as numbers in the case of dichotomous variables. For dichotomous outcomes, we calculated the odds ratio (OR) or risk ratio (RR) with 95% confidence interval (CI) and mean difference (MD) with 95% CI for continuous outcomes. If medians (IQR) were reported and the sample size was large enough, we could consider the median as equal to the mean and SD equal to IQR/1.35, otherwise the data were not collected []. The Mantel-Haenszel and inverse-variance test was used to analyze dichotomous outcomes and continuous variables among pooled studies, respectively. The I2 value was considered as an indicator of heterogeneity, and if >50%, it was considered statistically significant heterogeneity evidence.
Heterogeneity assumption was also measured by p value. p ≤ 0.10 indicated statistically significant heterogeneity, and the random effects model was selected for statistical analysis, otherwise a chosen fixed effects model was selected if p > 0.10. The subgroup analysis was performed for further evaluation. We carried out subgroup analysis according to different tidal volume (low or high) and inclusion criteria (PaO2/FiO2 ≤200 or others). If there was substantial heterogeneity in the outcomes, the Hartung-Knapp-Sidik-Jonkman (HKSJ) method would be used for further analysis.
CIs were calculated and presented in forest plots. Funnel plot analysis was performed when there were up to 10 enrolled studies. Publication bias was evaluated by Egger’s test using primary outcomes. Sensitivity analysis was conducted by removing each study in an orderly manner and re-analyzed again in order to distinguish potentially high-impact studies. Statistical analyses were performed with Review Manager 5.3 (RevMan, The Cochrane Collaboration, Oxford, UK), GRADEpro (McMaster University, Hamilton, ON, Canada, 2014), Stata version 14.0 (StataCorp) and RGui version 3.5.0.
Results
Description of Studies
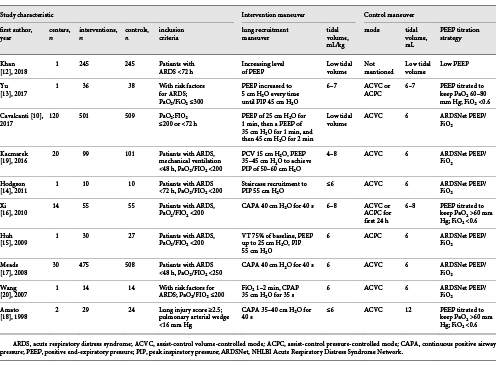
The process of literature selection is listed in Figure 1. We identified 524 potentially relevant studies (Pubmed 82, Embase 118, Ovid Medline 190, Cochrane Library database 134, Google Scholar resources 0). After carefully screening, 514 articles were eliminated for various reasons, such as duplicated publications (212), animal research (40), case report or reviews (36), protocol or conference abstracts (38), cross-trials or others (226). Finally, 10 RCTs and 3,025 patients were included [, -]. The basic characteristics, as well as LMRs and co-interventions in the enrolled studies are described in Table 1. In enrolled trials, recruitment maneuvers varied widely. For primary outcomes, 9 out of 10 trials reported in-hospital mortality [, -] and 28-day mortality [, , , -].
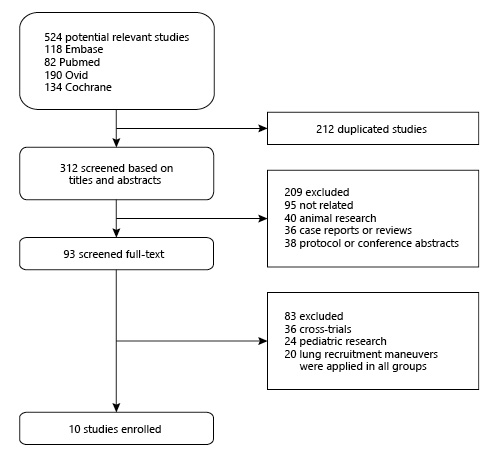
Fig. 1
Flowchart of selecting process about this meta-analysis.
Quality Assessment
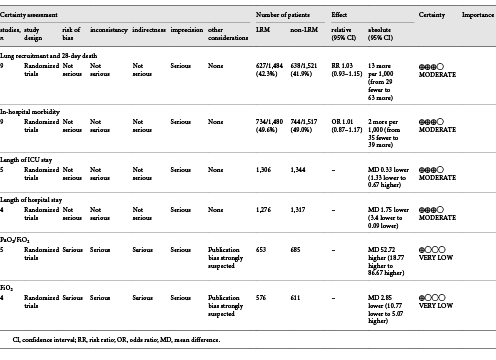
Quality assessment by Review Manager 5.3 and GRADEpro is shown in Figure 2a, b and Table 2. Eight out of 10 presented a low risk of random sequence generation [, -], and 7 of 10 showed a low risk of allocation concealment by describing the randomized method in detail [, , , -]. Although none of the RCTs had blinded investigators, the outcomes were not influenced by the lack of blinding. No evidence of publication bias was detected by Egger’s test (p = 0.089 and p = 0.917 for 28-day mortality and in-hospital mortality, respectively) in Stata version 14.0.
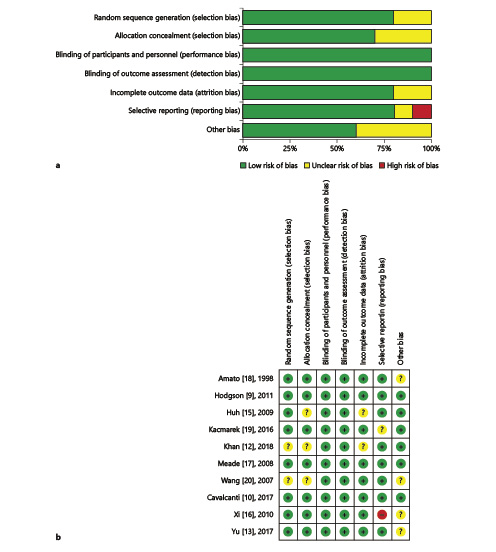
Fig. 2
Assessment of risk bias for RCTs. a A graph with percentages for all included studies. b A summary of bias for each included study.
Primary Outcomes
In-Hospital Mortality
Nine RCTs including 2,997 patients reported data on the in-hospital mortality with overall incidence of 49.3% (734/1,480 in LRM group, 744/1,517 in non-LRM group). There was no significant difference between the two groups using the fixed effect model (OR = 1.01; 95% CI, 0.87–1.17; p = 0.86), with moderate heterogeneity (χ2 = 14.6, I2 = 45%) (Fig. 3).
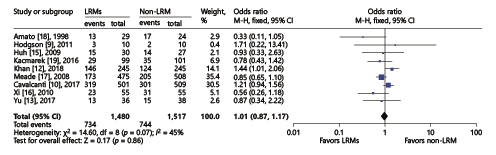
Fig. 3
Forest plot for in-hospital mortality between the LRM and non-LRM groups.
28-Day Mortality
Revman. In 9 RCTs including 3,005 subjects, 28-day mortality was reported. The 28-day mortality did not show any difference in the two groups with the random effects model either (RR = 0.92; 95% CI, 0.78–1.08; p = 0.3) with high heterogeneity (χ2 = 17.47, I2 = 54%) (Fig. 4). In order to reduce heterogeneity in the study design or intervention maneuver, we conducted the subgroup analysis as follows:
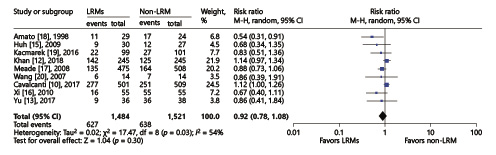
Fig. 4
Forest plot for the overall 28-day mortality between the LRM and non-LRM groups.
1 according to different inclusion criteria: PaO2/FiO2 <200 mm Hg or others;
2 compared with different control group: low tidal volume or high tidal volume.
There were 5 trials including 1,405 patients with PaO2/FiO2 <200 mm Hg [, , , , ]. The data were pooled from these trials and compared with the 4 other trials with various inclusion criteria [, , , ], but still presented high heterogeneity. Hence, we performed another kind of subgroup analysis with a different control group (Fig. 5a), as the enrolled study compared different tidal volumes in control groups (Fig. 5b). Notably, the result of 28-day mortality did not show any obvious statistical difference after subgroup analysis of the LRM and non-LRM groups.
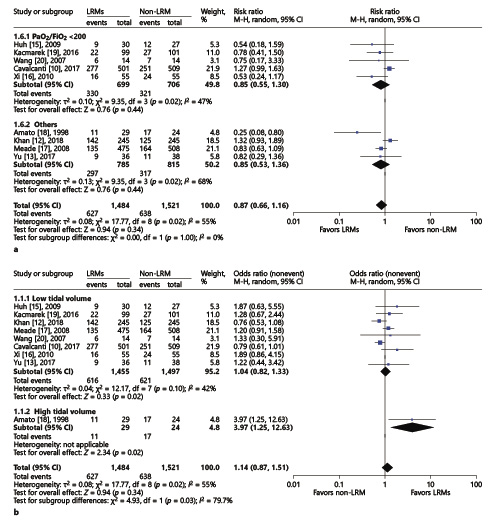
Fig. 5
Forest plot for subgroup analysis of the 28-day mortality between the LRM and non-LRM groups. a According to different inclusion criteria: PaO2/FiO2≤200 or others. b According to tidal volume in control group: low or high. τ
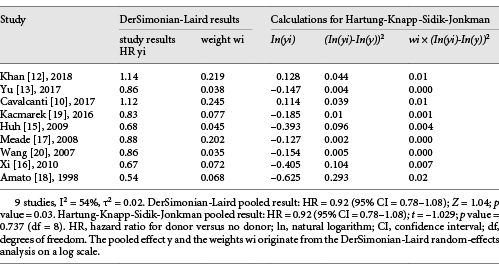
The HKSJ Method. Because of significant heterogeneity in 28-day mortality, the HKSJ method was performed to analyze the outcome. We converted DerSimonian-Laird (DL) results into HKSJ results for a logarithm based on outcome hazard ratio. Surprisingly, the 28-day mortality did not show any heterogeneity (p = 0.737) (Table 3).
Secondary Outcomes
In this updated meta-analysis, five secondary outcomes were compared, including the length of ICU stay, the length of hospital stay, PaO2/FiO2, and tidal volume. However, the PaO2/FiO2and tidal volume values varied greatly at different time points, and we pooled all the data on the third day. In one trial, 2- to 7-day data were chosen in the order of preference as the third-day data were not reported [].
The Length of ICU and Hospital Stay
Five trials presented the length of ICU stay [, , -], and another 4 reported the length of hospital stay [, , , ]. We conducted the analysis using the inverse-variance method and random effects. The final results showed that LRMs significantly reduced the length of hospital stay (MD = –1.75; 95% CI, –3.40 to –0.09; p = 0.04; p for heterogeneity = 0.3, I2 = 18%, Fig. 6), but had no influence on the length of ICU stay (MD = –0.33; 95% CI, –1.33 to 0.67; p = 0.52; p for heterogeneity = 0.46, I2 = 0%, Fig. 7).
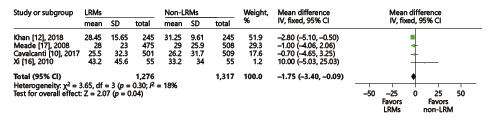
Fig. 6
Forest plot for the length of hospital stay between the LRM and non-LRM groups.
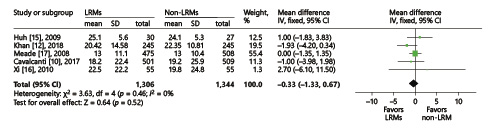
Fig. 7
Forest plot for ICU stay between the LRM and non-LRM groups.
PaO2/FiO2and FiO2
Five trials presented the PaO2/FiO2 ratio [, -], while 4 trials reported the tidal volume [, , , ] on the third day. We conducted the analysis using the inverse-variance method and random effects. Compared to non-LRM, the LRMs significantly increased the PaO2/FiO2 ratio (MD = 52.72; 95% CI, 18.77–86.67; p = 0.002; p for heterogeneity <0.001, I2 = 99%, Fig. 8), but with extremely high heterogeneity. Four trials reported FiO2 on the third day. Due to the overall analysis with high heterogeneity, we conducted subgroup analysis according to the sample size (large or small; ≥100 or <100). In a small sample size group with three trials [, , ], there was no significant difference in oxygen requirement between LRM and non-LRM groups (MD = 0.48, 95% CI, –2.44 to 3.39; p = 0.75; p for heterogeneity 0.96, I2 = 0%) (Fig. 9). Only one trial existed in the large sample size group that demonstrated that LRMs could reduce the oxygen requirement for patients with ARDS (p < 0.01) [].
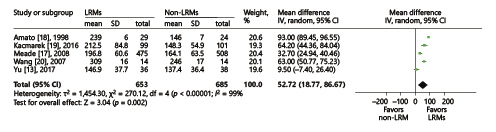
Fig. 8
Forest plot for PaO2/FiO2 ratio between the LRM and non-LRM groups.
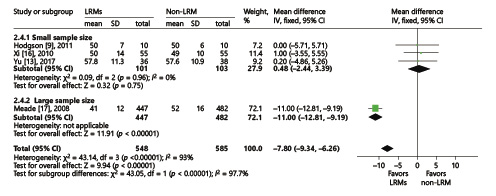
Fig. 9
Forest plot showing the subgroup comparison of FiO2 between the LRM and non-LRM groups.
Discussion
We included 10 trials that presented at least one of the primary outcomes in the updated meta-analysis. In ARDS patients, the results from our study proved that LRM neither decreased the mortality nor reduced the ICU and hospital stay, but it could improve oxygenation on the third day and reduce the length of hospital stay. However, our findings contradicted a previous meta-analysis which reported the advantages of LRMs in mortality []. The underlying explanation for this contradiction was that more recent studies were included. One of them was a multicenter research with a large sample size [], while the other had just one center with adequate sample size [], and they played pivotal roles in obtaining results. Their findings did not support routine use of LRMs in ARDS patients [, ], which had conclusions similar to ours. To our knowledge, people trusted a large RCT over a small one, because it had less risk of bias and provided more reliable results. On the other hand, Goligher et al. [] included one study without lung protective ventilation strategy. Based on the existing literature, large tidal volume could be considered as an independent risk factor for mortality in ARDS patients []. Compared to 12 mL kg–1, the ARDSNet trial reported that 6 mL kg–1 had a beneficial effect on the survival rate of ARDS patients []. Obviously, the majority of our enrolled studies excluded this interference factor, except for one []. To minimize the risk of bias, we conducted the subgroup analysis according to different tidal volume.
In a retrospective study, the author found that a PaO2/FiO2ratio <100 significantly indicated high 28-day mortality, and over 200 showed a downward trend []. Therefore, all the trials were divided into two subgroups according to the different PaO2/FiO2 ratios and tidal volume, and then reanalyzed. The results demonstrated that compared to non-LRM, there was no significant difference with respect to in-hospital and 28-day mortality with the application of LRMs, even performing subgroup analysis. Although the results showed moderate heterogeneity that was acceptable, the different protocols of LRMs and co-interventions with different levels of PEEP cannot be ignored, as they could be the potential confounding factors leading to heterogeneity.
Interestingly, although sub-group analysis was performed, the substantive heterogeneity was still significant. The DL method was the most commonly used method for a random effects meta-analysis, which was also used in Revman []. However, this method was suboptimal when the sample size was small and had moderate or substantial heterogeneity [, ]. Given this deficiency, the HKSJ method has chosen for further analysis in our study. Emerging evidence has shown that the HKSJ method performed better than DL, especially when there is unexplained heterogeneity and the number of enrolled studies is small [, ]. IntHout et al. [] compared the HKSJ and DL methods and found that the former resulted in more adequate error rates in meta-analysis of small sample sizes. In our meta-analysis, we found there was no significant heterogeneity in 28-day mortality using the HKSJ method. This difference may be attributed to the mean error rates of the DL approach, which were consistently higher than those of the HKSJ approach when there was heterogeneity, although the latter also doubled to 10% in scenarios with only one large trial [].
One of the main pathologic changes in ARDS is alveolar collapse or atelectasis that leads to effective ventilation reduction and poor oxygenation []. Recently, LRMs have been applied for improving oxygenation in patients with ARDS, though their benefits have not yet been determined. Zhao et al. [] suggested that patients’ SpO2, PaO2/FiO2, and Qs/Qt improved significantly after LRMs, but Yun et al. [] found that not all patients with optimally recruited lung tissue during LRMs showed significant improvement of oxygenation, which was assessed by electrical impedance tomography. There were many reasons that led to these contrasting results. It is quite well known that individual lung physiological characteristics vary greatly, as well as the details of LRM among enrolled studies. Five trials reported the PaO2/FiO2 ratio on the third day. The results revealed that the PaO2/FiO2 ratio improved with LRMs, a finding similar to Hodgson’s review [] except at different time points. Accordingly, the overall oxygen requirement was listed to have high heterogeneity. Nevertheless, during subgroup analysis, we found that the oxygen requirements showed a great difference between small and large sample sizes, which may predict selection bias (publication or reporting bias) and poor methodological quality of smaller studies (design, analysis, fraud). Furthermore, mechanical ventilation was widely applied in ARDS patients, and it has become the cornerstone in ARDS management. A national survey showed that vecuronium was routinely used in 18.6% and frequently in 30.5% of ICU patients in the USA []. Rhoney and Murry [] showed that the use of neuromuscular blocking agents was prolonged beyond 72 h in 10–20% of ICU patients. Early use of neuromuscular blocking agents was an important factor because the difference in PaO2/FiO2 ratio and better oxygenation on the third day could not be considered significant without knowing whether all studies advocated muscle paralysis and duration. Papazian et al. [] reported the benefit of early administration of neuromuscular blocking agents on the outcomes of ARDS patients. Gainnier et al. [] reported a significantly beneficial effect on the PaO2/FiO2 ratio in the neuromuscular blocking agent-treated group of patients compared to the control group. This became especially important as the PaO2/FiO2 ratio had been compared and the muscle paralysis could have been a confounding factor here. Unfortunately, by screening all enrolled research, we found that the use of neuromuscular blocking agents had not been mentioned. Hence, the conclusions must be interpreted cautiously. Even with the singular maneuver, frequency and pressure of recruitment have an extreme influence on the outcomes. Additionally, due to different measurements, neuromuscular blocking agents used, and ventilation patterns, it was true that recruited studies presented low homogeneity.
Limitations
There are some limitations in our meta-analysis. With regard to all enrolled RCTs, the majority of LRMs were combined with other interventions (titration PEEP, different modes of ventilation, and low tidal volume), which signified that any difference in outcomes between LRM and non-LRM groups could be attributed to the multiple effect of the interventions or, in other words, to the combined synergistic effects of two or more of these components. Thus, the conclusions were not convincing enough. Only one trial allowed recruitment maneuver as the single intervention without PEEP and showed no difference between the two groups, but the quality assessment revealed a moderate risk of bias []. In the future, well-designed multigroup RCTs are needed to provide high-quality evidence.
Conclusion
A recruitment maneuver is regularly performed in clinical practice as part of a lung protective strategy in order to re-expand collapsed lung tissue. Existing evidence supports the use of the recruitment maneuver combined with PEEP to improve oxygenation on the third day and reduce the length of hospital stay for patients with ARDS, but without beneficial effects on mortality. However, there has been no consensus on the ideal recruitment strategy. Further research is required to explore the efficacy and feasibility of recruitment therapy with respect to frequency, peak inspiratory pressure, optimal PEEP, suitable FiO2, and the use of neuromuscular blocking agents. More research should focus on distinguishing the benefits of recruitment maneuvers rather than multiple exposures. Moreover, optimal analysis methods should be chosen for analysis in order to obtain real outcomes.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was carried out on the support of the Department of Anesthesiology, Chengdu Women’s and Children’s Central Hospital. The sponsor did not take part in the preparation, design, conducting, and writing of the manuscript.
Author Contributions
Conceptualization: Yu Cui, Rong Cao, Yu Wang.
Data retrieval: Yu Cui, Yu Wang, Rong Cao.
Project administration: Gen Li.
Supervision: Yu Cui.
Validation: Rong Cao.
Writing – original draft: Yu Cui, Yu Wang.
Writing – review and editing: Rong Cao, Yu Cui.
References
- 1. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler AAcute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
- 2. Carrasco Loza R, Villamizar Rodríguez G, Medel Fernández N. Ventilator-Induced Lung Injury (VILI) in Acute Respiratory Distress Syndrome (ARDS): Volutrauma and Molecular Effects. Open Respir Med J. 2015;9Suppl 2: M6:112–9.
- 3. Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–73.
- 4. O’Gara B, Fan E, Talmor DS. Controversies in the Management of Severe ARDS: Optimal Ventilator Management and Use of Rescue Therapies. Semin Respir Crit Care Med. 2015;36(6):823–34.
- 5. Tusman G, Böhm SH, Sipmann FS, Maisch S. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg. 2004;98(6):1604–9.
- 6. Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians. 1998;110(6):482–8.
- 7. Fan E, Checkley W, Stewart TE, Muscedere J, Lesur O, Granton JT, et al Complications from recruitment maneuvers in patients with acute lung injury: secondary analysis from the lung open ventilation study. Respir Care. 2012;57(11):1842–9.
- 8. Goligher EC, Hodgson CL, Adhikari NKJ, et al Lung Recruitment Maneuvers for Adult Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. 2017; 14(Supplement_4):S304-S311. doi: ATS. 201704-340OT.
- 9. Hodgson C, Goligher EC, Young ME, Keating JL, Holland AE, Romero L, et al Recruitment manoeuvres for adults with acute respiratory distress syndrome receiving mechanical ventilation. Cochrane Database Syst Rev. 2016;11:CD006667.
- 10. Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DM, Damiani LP, Guimarães HP, et alWriting Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–45.
- 11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
- 12. Khan NA, Saleen M, Ashfaq A, Yusuf M. Is the lung recruitment and titrated positive end expiratory pressure a better strategy as compare to low PEEP on mortality in patients with acute respiratory distress syndrome. Medical Forum Monthly.2018;29:93–7.
- 13. Yu S, Hu TX, Jin J, Zhang S. Effect of protective lung ventilation strategy combined with lung recruitment maneuver in patients with acute respiratory distress syndrome (ARDS). J Acute Dis. 2017;6(4):163–8.
- 14. Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, et al A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15(3):R133.
- 15. Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13(1):R22.
- 16. Xi XM, Jiang L, Zhu BRM group. Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl). 2010;123(21):3100–5.
- 17. Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et alLung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–45.
- 18. Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–54.
- 19. Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, et alOpen Lung Approach Network. Open Lung Approach Network. Open lung approach for the acute respiratory distress syndrome: a pilot randomized controlled trial. Crit Care Med. 2016;44(1):32–42.
- 20. Wang XZ, Lü CJ, Gao FQ, Li XH, Hao D, Ning FY. [Comparison of the effects of BiPAP ventilation combined with lung recruitment maneuvers and low tidal volume A/C ventilation in patients with acute respiratory distress syndrome]. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(1):44–7.
- 21. Walkey AJ, Goligher EC, Del Sorbo L, et al Low tidal volume versus non-volume-limited strategies for patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4): S271-S279. doi: .
- 22. Azoulay E, Lemiale V, Mourvillier B, Garrouste-Orgeas M, Schwebel C, Ruckly S, et alOUTCOMEREA Study Group. Management and outcomes of acute respiratory distress syndrome patients with and without comorbid conditions. Intensive Care Med. 2018;44(7):1050–60.
- 23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
- 24. Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–89.
- 25. Hartung J, Knapp G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat Med. 2001;20(12):1771–82.
- 26. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25.
- 27. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et alLUNG SAFE InvestigatorsESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
- 28. Zhao P, Yang J, He Y. Analysing the therapeutical action of lung recruitment maneuver on patients with acute respiratory distress syndrome by comparing different ventilation strategies. Biomedical Research (India). 2017;28(4):1828–31.
- 29. Yun L, He HW, Möller K, Frerichs I, Liu D, Zhao Z. Assessment of Lung Recruitment by Electrical Impedance Tomography and Oxygenation in ARDS Patients. Medicine (Baltimore). 2016;95(22):e3820.
- 30. Rhoney DH, Murry KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med. 2003;18(3):139–45.
- 31. Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et alACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–16.
- 32. Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32(1):113–9.
Y.C., R.C., and Y.W. contribute equally ton this article and share first authorship.