Introduction
Disgust is a disease-avoidance emotion affecting several aspects of life, as it may influence how we select what we eat, our friends, sexual partners, what social group we adhere to, the clothing we wear, the music we listen to and, probably, our concept of morality (; ; ). Despite the 140 years that have elapsed since Charles Darwin published his influential work, The Expression of the Emotions in Man and Animals (), our understanding of the psychobiology of disgust has progressed rapidly only in the last decades.
Studies have revealed that disgust processing is mediated by a complex network of cortical (including insular, cingulate and orbitofrontal cortex) and subcortical regions (e.g. the striatum) implicated in the processing of reward and aversion. Regions within this network are recruited when experiencing unpleasant odors and liquids (; ; , ), and when feeling nausea (; ) but also during imaginative experiences of disgust, e.g. when watching or imagining disgusting foods and scenarios (; ; ). Moreover, regions like the cingulate cortex and the insula are also vicariously active when watching disgusted expressions (; ; ; ; ), suggesting that perception of disgust in others may tap into shared emotion representations between self and other (; ; ; ). Such overlapping activations for one’s own experience of disgust and the disgusted expressions of others may also reflect the emotional and homoeostatic salience of first- and third-person experiences of disgust. The insula and the cingulate cortex are recruited in a wide variety of tasks involving the subjective awareness of both positive and negative feelings (; ; ; ; ; ; ; ) and could thus play a domain-general role, that is, to identify the most salient among several internal and extrapersonal stimuli in order to guide behavior. Interestingly however, electrical stimulation of the insular cortex can induce genuine disgust-related sensations (i.e. nausea, unpleasant tastes and sensations in the mouth and stomach) and corresponding orofacial motor responses (; ; ), pointing to disgust-specific mechanisms in the insula and its strong link with motor circuits controlling oral motor behavior (; ).
Indeed, the insula and other regions that are recruited when sensing or imagining disgust possess reciprocal connections with a number of motor areas, including somatomotor orofacial regions (; ; ; ; ; ) that control ingestion through several descending cortico-bulbar pathways. These include cortico-pharyngeal and cortico-vagal pathways that regulate swallowing (; ) and cortico-hypoglossal projections controlling the tongue, which is the primary organ of taste in the gustatory system and plays a major role in the preparatory oral activity that precedes swallowing (). Remarkably, the tongue has a major role not only in favoring swallowing but also in preventing it, e.g. when tasting disgusting and potentially toxic food. In these circumstances, ingestion has to be inhibited in advance to prevent contamination from toxic and potentially lethal substances.
However, little is known about the neurophysiological mechanisms underlying motor reactions to the sight of potentially toxic foods. Moreover, no studies have tested whether the human motor system undergoes similar neurophysiological modulations when vicariously sensing distaste in others. To address this issue, in this study we used transcranial magnetic stimulation (TMS) to monitor changes in primary motor cortex (M1) excitability while participants watched disgust-related and control pictures. These included pictures representing gustatory disgust/revulsion (namely, spoiled foods and facial expressions of distaste), non-gustatory disgust (i.e. disgusting invertebrates inducing repulsion, such as worms), corresponding emotionally positive stimuli (i.e. fresh foods, facial expressions and invertebrates) and neutral control stimuli (fixation cross). This way, we investigated the corticomotor correlates of imaginative and social observational aspects of gustatory and non-gustatory disgust. We targeted the tongue representation in M1 (tM1) and recorded motor-evoked potentials (MEPs) from the apex of the tongue as a measure of tM1 cortico-hypoglossal excitability. As a non-oral somatotopic control, we also targeted the arm representation in M1 (aM1) and recorded MEPs from the extensor carpi radialis (ECR) as a measure of aM1 cortico-spinal excitability.
TMS-induced MEPs are an established method for investigating the excitability of the human motor system during emotion processing (, ; ; ). Previous TMS studies have shown that processing salient and emotionally threatening stimuli is often associated with inhibition of corticomotor output (; ; ). For example, viewing social cues of potential threats (i.e. a fearful body posture) was found to rapidly freeze grasping-related cortico-spinal circuits, suggesting an automatic suppression of approach tendencies (). Similarly, pain administered on one’s own body part reduces the excitability of motor circuits controlling that body part () and watching pain in others triggers similar body part-specific motor inhibition (, ; ). However, to date, no study has specifically tested whether similar somatotopic inhibitory modulations occur at the level of tM1 cortico-hypoglossal projections during imaginative and social observational aspects of disgust.
Based on the above-mentioned evidence of somatotopically specific motor inhibition when processing noxious or threatening stimuli and the specific role of the tongue in preventing ingestion of contaminants, we hypothesized that being exposed to rotten foods eliciting distaste and revulsion might specifically reduce tM1 cortico-hypoglossal excitability (but not aM1 cortico-spinal excitability). Because of the strong link between personal experience, imagery and social perception of emotions (e.g. ; ; ; ), we also predicted a similar ‘vicarious’ tM1 suppression when watching facial expressions of gustatory disgust. No similar tM1 modulations were expected for disgusting invertebrates—which are less related to distaste and revulsion, and more to repulsion and physical withdrawal ().
Moreover, based on the evidence that brain activations during perception of disgusting stimuli are influenced by inter-individual differences in disgust sensitivity (; ; ; ), we predicted that greater disgust sensitivity would be associated with stronger suppression of tM1 cortico-hypoglossal output during perception of gustatory disgust.
Materials and methods
Participants
Sixteen healthy participants (eight males, mean age ± SD: 22.93 years ± 2.23) with no contraindications to TMS () took part in the study. Twelve participants were tested at the University of Bologna, while four participants were tested at the University of Queensland by the same researcher. All subjects had normal or corrected-to-normal visual acuity and gave their written informed consent. The experimental procedures were approved by the local ethics committees and were carried out in accordance with the principles of the 1964 Helsinki Declaration.
Visual stimuli
The experimental stimuli consisted of three categories of pictures (subtending a visual angle of 18.53 × 12.19° and with comparable luminance; Figure 1) presented in separate blocks. One block included pictures of disgusting foods (i.e. moldy/rotten foods that can elicit distaste and revulsion), while pictures of fresh food and a fixation cross were used as emotionally positive and neutral controls. Another block included pictures of facial expressions of gustatory disgust, i.e. facial reactions of distaste/revulsion for unpleasant tastes (showing a gaping mouth and protruded tongue), while a fixation cross and pleasant facial expressions were used as control stimuli. A third block included disgusting stimuli not associated with distaste/revulsion (i.e. worms and cockroaches, which can elicit repulsion and withdrawal tendencies but not gustatory disgust), while a fixation cross and positive-looking butterfly and ladybug pictures were used as control stimuli.
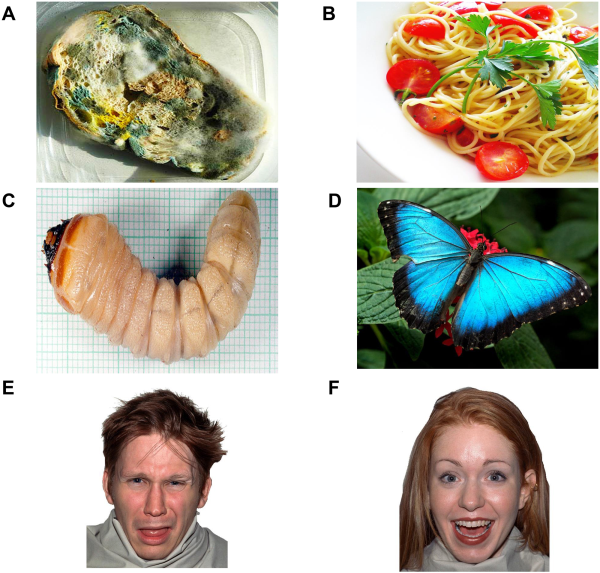
Fig. 1
Disgust-related and positive visual stimuli. The figure shows examples from the food, invertebrate and face categories that were presented in different blocks: ‘A’ disgusting food; ‘B’ positive food; ‘C’ disgusting invertebrate; ‘D’ positive invertebrate; ‘E’ disgusted expression; ‘F’ positive expression. In each food, invertebrate and face block, participants were also exposed to a fixation cross (neutral control).
Facial expressions were selected from the Nimstim set (), whereas food and invertebrate stimuli were selected from a picture database on the internet. We carried out a pilot study to select disgusting pictures with comparable disgust and arousal ratings across the three categories (Supplementary Materials and Supplementary Table S1). However, in a second pilot, when we distinguished between two different aspects of disgust, namely distaste/revulsion and repulsion/withdrawal, we found the former aspect to be more associated with gustatory disgust stimuli (particularly rotten foods, but also expressions of distaste) and the latter with disgusting invertebrates (Supplementary Table S2). In the second pilot study, we also ensured that the disgust-related pictures and the corresponding positive pictures had comparable arousal ratings but opposite valence (Supplementary Table S2).
Within each block, we used fixation crosses as emotionally neutral stimuli. We chose fixation crosses because some ‘neutral’ stimuli, e.g. neutral facial expressions, are often perceived as ambiguous rather than emotionally neutral (; Toki et al., 2012). Even more critically, the use of the same neutral stimulus across the three blocks allowed us to monitor any possible changes in excitability over time.
Electromyogram recording and TMS
Electromyogram (EMG) recording was performed with a Biopac MP 150 electromyograph (University of Bologna) or a Grass P511 isolated amplifier (University of Queensland). The EMG signal was sampled at 10 kHz, band-pass filtered (20 Hz–2.5 kHz) and stored for offline analysis.
Two different electrodes montages were used for recording MEPs from the target (tongue) and control (ECR) muscles (; ). For the tongue, we used Ag-AgCl electrodes (1 cm diameter) mounted on a 1 × 1 cm plastic plate and fixed on a metal clip device (). Though the cortical representation of the tongue muscles is mainly bilateral (; ), it is debatable whether unilateral tongue motor responses can be safely recorded devoid of volume-conducted potentials from the contralateral side of the tongue (; ). Thus, we decided to record the tongue on the midline, instead of a unilateral recording. Accordingly, the active and reference electrodes were placed on the dorsal and ventral aspects of the tongue, respectively, ∼1.5 cm caudal to the tongue apex. For the ECR, pairs of Ag-AgCl surface electrodes (1 cm diameter) were placed over the muscle belly (active) and dorsal wrist (reference). For both tongue and ECR, ground electrodes were placed over the elbow. TMS was administered using a 70 mm figure-of-eight focal coil connected to a Magstim Rapid2 (University of Bologna) or a Magstim 200 (University of Queensland) stimulator. The coil intersection was placed tangentially to the scalp to induce current flows in a posterior–anterior direction with both stimulator types.
Stimulation of tongue (tM1) and ECR (aM1) motor representations from the same scalp site was not possible. Thus, we performed two separate stimulation sessions whose order was counterbalanced. From tM1 and aM1 optimal scalp positions (OSP, i.e. the stimulation positions that induce MEPs of maximal amplitude from the corresponding muscle), the resting motor threshold (rMT) was defined as the lowest intensity of stimulation that produced five MEPs with an amplitude > 50 μV on 5 out of 10 consecutive pulses (). During the experimental conditions, MEPs were elicited by stimulating the OSP with an intensity of 120% of rMT and stored on a computer for offline analysis.
Procedure
Participants were comfortably seated in front of a computer screen. Each stimulation session (Tongue, ECR) consisted of three 48-trial blocks (food, invertebrates, faces) whose order was randomized. In each block, 16 MEPs were recorded during presentation of a fixation cross (8 MEPs at the beginning and 8 MEPs at the end of the block) and the remaining 32 MEPs were recorded during the presentation of emotional stimuli (i.e. 4 stimuli × 4 repetitions × 2 emotion categories: disgusting vs positive). In each trial, a fixation cross was presented at the center of the screen for 1 s. In the fixation trials, the cross remained on the screen for another 2 s. In the emotional stimulus trials, one picture was presented for 2 s. During cross/picture presentation, a single pulse of TMS was administered over either the tM1 or aM1, and a MEP was recorded from the corresponding target muscle. TMS was delivered at random times ranging between 1100 and 1400 ms after cross/picture onset to avoid any priming effects that might influence MEP amplitude (Vicario et al., , ; ). The inter-stimulus interval was 7000 ms. The inter-pulse interval was > 10 s to avoid changes in motor excitability due to TMS per se (). The lack of change was confirmed by comparing MEPs during the fixation baseline across block orders (Supplementary Materials).
To maintain attention and check picture recognition, in six vigilance trials (two per block), subjects were asked to verbally describe the last picture they saw. To avoid changes in excitability due to preparation of verbal responses (; ), participants were instructed to provide their response about 2-3 s after the release of the magnetic pulse (; ). All participants correctly described the pictures in all the vigilance trials.
Finally, we tested inter individual differences in susceptibility to the experience of disgust by using the Disgust Scale (DS) (), the most widely used self-report scale for assessing disgust propensity. We used the revised version of the DS-R recommended by . This version includes 13 true–false items (scored 0 or 1) where participants are asked to report their agreement with a statement (e.g. ‘Even if I was hungry, I would not drink a bowl of my favorite soup if it had been stirred by a used but thoroughly washed flyswatter’) and 12 items that are rated on a 3-point scale (scored 0, 0.5 and 1) that assesses the extent to which participants find a given experience (e.g. ‘you are about to drink a glass of milk when you smell that it is spoiled’) not disgusting at all, slightly disgusting or very disgusting. We computed a global score of disgust sensitivity (range 0–25) by summing responses to the 25 items (DS-R) ().
Data analysis
Two participants were not able to complete the experimental session, due to mild discomfort during tM1 stimulation. Therefore, the analysis of tongue MEPs was conducted on 14 participants. Due to a technical failure during MEP recording, ECR data from one participant were lost and, thus, the analysis of ECR MEPs was conducted on thirteen participants. Trials with EMG activity prior to TMS were discarded from the analysis (11.5%). Mean MEP amplitude values in each condition were measured peak-to-peak (in mV). Outlier values (±2.5 SD of the mean) were identified in each condition and removed (2.5%). Logarithmic transformation was applied to raw amplitude values [log (mean MEP amplitude value + 1)] in order to reduce skewness and inter individual variability. Because the Shapiro-Wilk test showed that the transformed data were not normally distributed, MEP amplitudes were analyzed by means of non-parametric Friedman analysis of variance (ANOVA) and Wilcoxon matched-pairs tests (see also Supplementary Materials). The significance level was set at P = 0.05.
A regression analysis was carried out to interpret any modulation of tM1 excitability when observing foods and facial expressions associated with gustatory disgust. We tested whether such a neurophysiological modulation would be predicted by inter individual differences in DS-R disgust sensitivity. To compute an index reflecting any modulation for gustatory disgust, we calculated a MEP contrast index as the difference in MEP amplitude between the disgust-related condition and the mean MEP amplitude in the two control conditions (positive versions of food and facial stimuli and fixation baselines) and then averaged the two differences. This index was entered as a dependent variable in a regression analysis with the DS-R scores as a predictor. Moreover, non-parametric correlations using the Spearman coefficient (ρ) were computed.
Results
The Friedman ANOVA performed on tongue MEPs was significant (χ28 = 17.45, P = 0.026), whereas the analysis on ECR MEPs was not (χ28= 12.98, P = 0.11), suggesting that visual conditions consistently modulated tM1 cortico-hypoglossal excitability but not aM1 cortico-spinal excitability (Figure 2). Further Friedman ANOVAs and Wilcoxon matched-pairs tests were performed to isolate significant tM1 modulations within the three experimental blocks. Friedman ANOVAs restricted to food (χ22 = 13.00, P = 0.0015) and face blocks (χ22= 10.71, P = 0.0047) were significant. Wilcoxon tests showed that MEP amplitudes from the tongue were lower for pictures of rotten foods (mean ± SD: 0.171 mV ± 0.156) than for pictures of pleasant foods (0.186 mV ± 0.162; Z= 2.17, P = 0.03) and the food block baseline (0.182 mV ± 0.158; Z= 3.30, P < 0.001), which in turn did not differ from one another (Z = 0.97, P = 0.33). Moreover, MEP amplitudes were lower for distaste expressions (0.122 mV ± 0.080) than for positive facial expressions (0.142 mV ± 0.091; Z= 2.73, P = 0.006) and the face block baseline (0.138 mV ± 0.090; Z= 2.92, P = 0.004) which in turn did not differ from one another (Z = 0.28, P = 0.78).
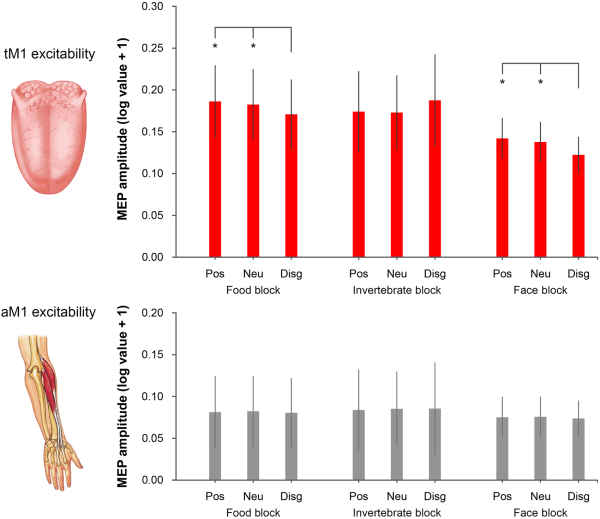
Fig. 2
MEP amplitudes recorded from the tongue (upper panel) and the ECR (lower panel). In the food block, tongue MEPs were reduced for disgusting foods relative to positive (pleasant food) and neutral (fixation cross) conditions. In the face block, tongue MEPs were reduced for faces expressing disgust relative to positive (happy expressions) and neutral (fixation) conditions. No difference in tongue MEPs was found in the invertebrate block. No difference in ECR MEPs was found for any of the three blocks. Error bars denote SEM. Asterisks (*) indicate P < 0.05.
This tM1 cortico-hypoglossal suppression was specific for visual stimuli associated with oral disgust, as Friedman ANOVAs (χ22 = 0.43, P = 0.81) and Wilcoxon tests (all Z < 0.45, P > 0.65) performed on the invertebrate block were not significant. Moreover, tM1 suppression was comparable in the two groups of participants tested at the University of Bologna and the University of Queensland (Supplementary Materials).
To estimate the suppression effect induced by pictures of disgusting food, we computed a MEP contrast index as the difference between the tongue MEP amplitude recorded during disgusting food pictures and the mean MEP amplitude recorded during pleasant food pictures and the food block baseline. A similar MEP contrast index was computed for disgusted facial expressions (distaste expressions minus mean of pleasant faces and the face block baseline) and invertebrates (disgusting minus pleasant invertebrates and the invertebrate block baseline). A Friedman ANOVA on these contrast indices was significant (χ28= 7.00, P = 0.03). Wilcoxon tests showed that MEP contrast indices for disgusting foods (−0.012 mV ± 0.008) and facial expressions of distaste (−0.015 mV ± 0.016) were comparable (Z = 0.72, P > 0.47) and lower than the MEP contrast index for disgusting invertebrates (0.014 mV ± 0.048; all Z > 2.29, all P < 0.022).
The same analysis performed on MEP contrasts computed from the ECR data showed no significant effects (Friedman ANOVA: χ22 = 0.46, P = 0.79; Wilcoxon tests: all Z < 0.17, all P > 0.86).
To test whether inter individual differences in disgust sensitivity (DS-R) predict the magnitude of tM1 inhibition during observation of oral-related disgust pictures (indexed by the mean MEP contrast for disgusting foods and facial expressions of distaste), a regression analysis was performed. Because the two variables were normally distributed (Shapiro-Wilk test: P > 0.24) a parametric analysis was used. The regression model was significant and it evidenced a negative relation with a large effect size (adjR2 = 0.31, β = −0.60, F1,12 = 6.83, P = 0.023; ƒ2 = 0.45) that was confirmed using non-parametric correlation (Spearman ρ = −0.54, P = 0.047). The model strongly improved after the removal of one outlier with standard residual > 2 SD (adjR2 = 0.55, β = −0.77, F1,11 = 15.82, P = 0.002; ƒ2 = 1.24; Figure 3).
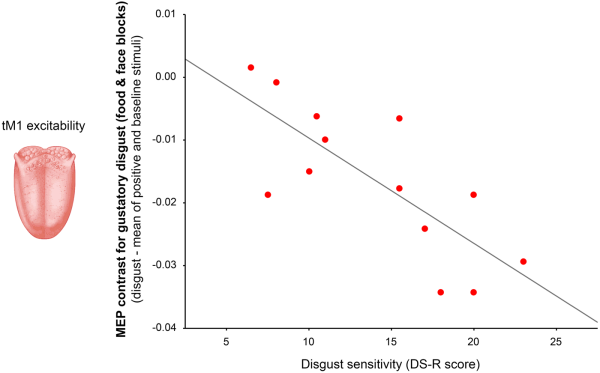
Fig. 3
Simple correlation between disgust sensitivity (measured by the DS-R score) and the magnitude of tM1 suppression detected when seeing pictures related to gustatory disgust (indexed by the mean MEP contrast for disgusting foods and facial expressions of distaste).
The negative relationship indicated that participants with greater disgust sensitivity tended to show stronger reduction in tM1 excitability when exposed to pictures related to oral disgust. The same regression analysis performed on the MEP contrast calculated from the ECR was not significant (adjR2 = 0.03, β = −0.0002, F < 1, P = 0.55; Spearman ρ = −0.13, P = 0.67; no outliers in the dataset).
Discussion
To establish the cortico-motor correlates of imaginative and social observational aspects of disgust, we measured tongue MEPs as an index of tM1 cortico-hypoglossal output during the observation of pictures related to gustatory disgust and revulsion—namely, rotten foods and facial expressions of distaste, as well as pictures eliciting non-oral disgust (i.e. repulsion, originating from disgusting invertebrates like worms) and control stimuli.
In line with our hypotheses, we detected a modulation of tM1 excitability for the food and face picture categories, which possess clear associations with the tongue, but not for the invertebrate category. In contrast, no modulations of aM1 cortico-spinal excitability were detected across stimulus categories. These findings hint at somatotopic specificity when processing visual signs of gustatory disgust. They suggest that tM1 cortico-hypoglossal projections might be sensitive not only to disgust and the imaginative aspects of disgust that are likely evoked by the sight of disgusting foods (Supplementary Tables S1 and S2)—and that may require inhibition of preparatory tongue activity—but also to the perception of social signals of gustatory disgust in others. In particular, we found that exposure to pictures of rotten foods brought about a consistent reduction in the amplitude of MEPs recorded from the tongue. Tongue MEPs in this condition were lower than when participants observed pictures of fresh food or a fixation cross (baseline). A similar reduction was found when watching pictures of faces expressing distaste, compared with pictures of happy faces and the baseline. No similar modulations were found when seeing disgusting invertebrates that are more associated with repulsion and physical withdrawal/not wanting to touch or be touched. This selectivity suggests that tM1 cortico-hypoglossal excitability is specifically affected by images related to gustatory revulsion but not by non-oral repulsion.
We propose that the suppression of tM1 cortico-hypoglossal excitability while seeing pictures of disgusting foods might reflect an implicit avoidant-defensive mechanism of motor inhibition to prevent the ingestion of potentially harmful contaminants. In this sense, one might interpret this physiological phenomenon as an index of anticipatory action inhibition that is triggered by the sight of potentially threatening foods, and may have the ultimate role of preventing any toxin swallowing and contamination (; ).
The idea that detecting potential threats taps into motor inhibitory mechanisms is in line with influential models of emotion perception (, ; ). Although behavioral studies have reported generalized freezing effects, like the reduction of body sway when viewing aversive pictures (e.g. ; ; ), TMS evidence has documented specific inhibitory defensive mechanisms. For example, reduced M1 cortico-spinal excitability was found during administration of noxious stimulation to a specific body part (e.g. ; ). Moreover, suddenly approaching objects () or sounds (; ) occurring near the body (i.e., within the peripersonal space; ) were found to trigger body-part specific cortico-spinal inhibition. More recently, it was shown that observing signals of potential threats specifically reduces the excitability of hand motor circuits controlling approaching movements like grasping (). However, all these studies have limited their investigation to upper limb motor excitability or postural sway and none of them have specifically investigated the emotion of disgust. Our data expand previous evidence by showing that observation of potentially threatening spoiled foods selectively inhibits the observers’ tM1 cortico-hypoglossal pathway, possibly to prevent the organism from toxin contamination via oral ingestion.
These findings complement those of a previous study in which tobacco-addicted patients were exposed to craving-inducing visual cues (i.e. pictures of cigarettes) (). In those conditions, increased tongue MEPs was found. Although preliminary, the report of and the present study suggest that changes in the excitability of the tM1 cortico-hypoglossal pathway may reflect a somatomotor marker of oral approach/avoidance.
In a similar fashion, the reduced tM1 excitability during exposure to faces expressing distaste might be accounted for by a mirroring phenomenon that associates the observation of disgusted expressions with a specific somato-gustatory self-experience (i.e. distaste) and the consequent motor response (i.e. inhibition of preparatory tongue activity to avoid swallowing). This is in agreement with the notion of shared neural representations for self-experience of an emotion and the observation of the same emotion in others (; ; ; ; ; ; ).
Strong evidence of shared neural mechanisms in the human motor system comes from studies showing that the sight of an action facilitates those motor circuits involved in making the same action (; ; ; ). Notably, this mirroring might occur at a relatively abstract level. Studies indicate that the observer's motor system reflects the encoding of the distal goal of the action, irrespective of the specific movements performed to achieve it (, ; ). A similar proposal has been put forward for the tendency to imitate others’ emotional expressions (i.e. facial mimicry, ), based on the evidence that people mimic an interpretation of others’ emotions and not the actual expressive movements they observe (; ; ). This is relevant for our study, where the actors' disgust was signaled by gaping mouth and protruded tongue, i.e. an active movement of the tongue. Previous studies have found that perception of tongue movements may facilitate tongue MEPs (; ; ). Remarkably, however we found tongue MEPs inhibition rather than facilitation for observed expressions of distaste. This reduction hints at the vicarious activation of tongue motor programs that mirror those activated by imaginative aspects of gustatory disgust (i.e. elicited by the sight of rotten food pictures), rather than the observed actor’s tongue movements. Thus, we expand previous evidence by showing that tM1 cortico-hypoglossal projections are modulated in a similar mirror-like fashion when one is exposed to stimuli potentially eliciting revulsion within oneself (pictures of spoiled foods) and when exposed to facial expressions of distaste. Moreover, our study supports the aforementioned notions that motor mirroring may reflect high-level aspects of observed behavior (i.e. the inferred action goal/emotion), rather than the low-level motor pattern (i.e. the specific movement) being observed (, ; ; ).
Finally, we found that the suppression of tM1 excitability was predicted by DS-R scores. That is, participants who showed higher sensitivity to disgust in everyday life also showed greater reductions in tM1 excitability when seeing oral-related disgust (pictures of disgust-eliciting foods and facial expressions of distaste). These findings are in line with studies demonstrating that interindividual differences in disgust sensitivity modulate disgust-related neural activations (; ; ; ). They are also in keeping with studies showing that the magnitude of vicarious activations is predicted by inter individual differences in personality and social attitudes (; ; , ; ). Importantly, these correlational data further demonstrate a specific link between observational tM1 modulations and the experience of disgust.
Previous investigations have shown that observation of negative scenes affects the excitability of cortico-spinal projections to upper limb muscles (e.g. ; ; ; ), although none of the previous studies have specifically tested disgust-related scenes. In the current study, we did not find changes in ECR MEPs (which reflect aM1 cortico-spinal excitability) for any of the three stimulus categories. This result supports our hypothesis of somatotopic specificity and suggests that pictures signaling oral-related disgust do not suppress motor excitability in general, but specifically in the tM1 cortico-hypoglossal pathway, which is a key sector of the motor system involved in avoiding ingestion of contaminants.
Our study has potential limitations that should be discussed. First, the sample size of our study is not large and the experiment was carried out in two laboratories. However, control analyses indicate comparable effect sizes in the two datasets (Supplementary Materials), suggesting the reliability of the findings. Second, it is well established that MEPs recorded from the tongue reflect mainly tM1 cortico-hypoglossal excitability (; ), yet stimulation of the tM1 can activate other descending cortico-bulbar pathways (i.e. the cortico-pharyngeal and cortico-vagal pathways that regulate swallowing) which may affect tongue MEPs due to volume-conducted potentials. Although this influence is expected to be minimal, future studies might simultaneously monitor the dynamics of different cortico-bulbar pathways involved in ingestion during imaginative and observational social aspects of distaste. Third, although the experimental stimuli were evaluated in two pilot studies, participants in the main TMS experiment did not provide subjective ratings of the visual stimuli or the magnetic pulses. This prevented us from exploring the relations between disgust judgments, TMS discomfort and motor excitability. Thus, future studies could test whether subjective ratings of disgust closely parallel tongue MEPs, in a way that is similar to the results of the regression analysis (Figure 3) reported in the present study. Lastly, while the lack of ECR MEP modulation speaks against a generalized effect of gustatory disgust on motor excitability, a null finding should be interpreted with caution. In keeping with the notion that emotions prime the body for action (, ; ; ) one may expect that threatening stimuli requiring active avoidance may trigger activity in the observer’s motor system. The lack of modulation in the present experiment might have resulted from the general emotional qualities of the stimuli or the minimal functional role of our control muscle in approach/avoidance movements. Indeed, we do not rule out that other sectors of the motor system may be modulated by disgusting stimuli. For example, observing highly disgusting pictures associated with strong repulsion and arousal (e.g. spiders, dirty toilets, mutilations) could be expected to modulate several upper limb motor representations. Future studies will directly test these possibilities.
In sum, our study shows that pictures of revulsion-eliciting foods and facial expressions of distaste selectively reduce the excitability of tM1 cortico-hypoglossal projections, and this tM1 suppression is predicted by inter individual differences in disgust sensitivity. Our study suggests an avoidant-defensive mechanism in human cortico-hypoglossal circuits, and its vicarious activation during social perception of others’ distaste. These findings support the notion of shared neural representations of disgust and suggest that inter individual dispositions to experience disgust strongly affect such neural representations. Thus, these findings support a role for the motor system in emotion-driven motor anticipation and social cognition.
Acknowledgements
This work was supported by FP7-PEOPLE-2012-IEF Program (GAN-328551) grant awarded to CMV and Cogito Foundation (R-117/13 and 14-139-R), MIUR (RBFR12F0BD) and Ministero della Salute (GR-2010-2319335) grants awarded to AA. We thank Welber Marinovic for his invaluable help in data collection and Stephan Riek for allowing full access to lab facilities.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Alipour M., Chen Y., Jürgens U. (2002). Anterograde projections of the motorcortical tongue area in the saddle-back tamarin (Saguinus fuscicollis). Brain and Behavioral Evolution, 60, 101–16.
- Avenanti A., Annela L., Serino A. (2012). Suppression of premotor cortex disrupts motor coding of peripersonal space. Neuroimage, 63, 281–8.
- Avenanti A., Minio-Paluello I., Bufalari I., Aglioti S.M. (2009a). The pain of a model in the personality of an onlooker: influence of state-reactivity and personality traits on embodied empathy for pain. Neuroimage, 44, 275–83.
- Avenanti A., Sirigu A., Aglioti S.M. (2010). Racial bias reduces empathic sensorimotor resonance with other-race pain. Current Biology, 20, 1018–22.
- Avenanti A., Annella L., Candidi M., Urgesi C., Aglioti S.M. (2013a). Compensatory plasticity in the action observation network: virtual lesions of STS enhance anticipatory simulation of seen actions. Cerebral Cortex, 20, 570–80.
- Avenanti A., Bueti D., Galati G., Aglioti S.M. (2005). Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience, 8, 955–60.
- Avenanti A., Candidi M., Urgesi C. (2013b). Vicarious motor activation during action perception: beyond correlational evidence. Frontiers in Human Neuroscience, 7, 185.
- Avenanti A., Minio-Paluello I., Bufalari I., Aglioti S.M. (2006). Stimulus-driven modulation of motor-evoked potentials during observation of others’ pain. Neuroimage, 32, 316–24.
- Avenanti A., Minio-Paluello I., Sforza A., Aglioti S.M. (2009b). Freezing or escaping? Opposite modulations of empathic reactivity to the pain of others. Cortex, 45, 1072–7.
- Azevedo T.M., Volchan E., Imbiriba L.A., . (2005). A freezing-like posture to pictures of mutilation. Psychophysiology, 42, 255–60.
- Bastiaansen J.A., Thioux M., Keysers C. (2009). Evidence for mirror systems in emotions. Philosophical Transaction of the Royal Society of Londond B: Biological Science, 364, 2391–404.
- Borg C., de Jong P.J., Renken R.J., Georgiadis J.R. (2013). Disgust trait modulates frontal-posterior coupling as a function of disgust domain. Social Cognitive Affective Neuroscience, 8, 351–8.
- Borgomaneri S., Gazzola V., Avenanti A. (2012). Motor mapping of implied actions during perception of emotional body language. Brain Stimulation, 5, 70–6.
- Borgomaneri S., Gazzola V., Avenanti A. (2014). Temporal dynamics of motor cortex excitability during perception of natural emotional scenes. Social Cognitive and Affective Neuroscience, 9, 1451–7.
- Borgomaneri S., Vitale F., Avenanti A. (2015c). Early changes in corticospinal excitability when seeing fearful body expressions. Scientific Reports, 5, 14122.
- Borgomaneri S., Vitale F., Gazzola V., Avenanti A. (2015b). Seeing fearful body language rapidly freezes the observer’s motor cortex. Cortex, 65, 232–45.
- Borgomaneri S., Gazzola V., Avenanti A. (2015a). Transcranial magnetic stimulation reveals two functionally distinct stages of motor cortex involvement during perception of emotional body language. Brain Structures and Functions, 220, 2765–81.
- Calder A.J., Beaver J.D., Davis M.H., van Ditzhuijzen J., Keane J., Lawrence A.D. (2007). Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. European Journal of Neuroscience 25, 3422–8.
- Calder A.J., Keane J., Manes F., Antoun N., Young A.W. (2000). Impaired recognition and experience of disgust following brain injury. Nature Neuroscience, 3, 1077–8.
- Candidi M., Vicario C.M., Abreu A.M., Aglioti S.M. (2010). Competing mechanisms for mapping action-related categorical knowledge and observed actions. Cerebral Cortex, 20, 2832–41
- Caruana F., Jezzini A., Sbriscia-Fioretti B., Rizzolatti G., Gallese V. (2011). Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Current Biology, 21, 195–9.
- Cattaneo L., Caruana F., Jezzini A., Rizzolatti G. (2009). Representation of goal and movements without overt motor behavior in the human motor cortex: a transcranial magnetic stimulation study. Journal of Neuroscience, 29, 11134–8.
- Cattaneo L., Maule F., Barchiesi G., Rizzolatti G. (2013). The motor system resonates to the distal goal of observed actions: testing the inverse pliers paradigm in an ecological setting. Experimental Brain Research, 231, 37–49.
- Cauda F., Costa T., Torta D.M., . (2012). Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage, 62, 343–55.
- Cauda F., D'Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. (2011). Functional connectivity of the insula in the resting brain. Neuroimage, 55, 8–23.
- Cerliani L., Thomas R.M., Jbabdi S., . (2012). Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human Brain Mapping, 33, 2005–34.
- Chen C.H., Wu T., Chu N.S. (1999). Bilateral cortical representation of the intrinsic lingual muscles. Neurology, 52, 411–3.
- Chen R., Classen J., Gerloff C., . (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology, 48, 1398–403.
- Coelho C.M., Lipp O.V., Marinovic W., Wallis G., Riek S. (2010). Increased corticospinal excitability induced by unpleasant visual stimuli. Neuroscience Letters, 481, 135–8.
- Coombes S.A., Tandonnet C., Fujiyama H., Janelle C.M., Cauraugh J.H., Summers J.J. (2009). Emotion and motor preparation: A transcranial magnetic stimulation study of corticospinal motor tract excitability. Cognitive Affective and Behavioral Neuroscience, 9, 380–8.
- Cooney R.E., Atlas L.Y., Joormann J., Eugène F., Gotlib I.H. (2006). Amygdala activation in the processing of neutral faces in social anxiety disorder?: Is neutral really neutral?. Psychiatry Research, 148, 55–9.
- Couto B., Sedeño L., Sposato L.A., . (2013). Insular networks for emotional processing and social cognition: comparison of two case reports with either cortical or subcortical involvement. Cortex, 49, 1420–34.
- Craig A.D. (2009). How do you feel–now? The anterior insula and human awareness. Nature Review Neuroscience, 10, 59–70.
- Dapretto M., Davies M.S., Pfeifer J.H., . (2006). Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience, 9, 28–30.
- Darwin C.R. (1872). The Expression of the Emotions in Man and Animals, 1st edn. London: John Murray
- D’Ausilio A., Jarmolowska J., Busan P., Bufalari I., Craighero L. (2011). Tongue corticospinal modulation during attended verbal stimuli: priming and coarticulation effects. Neuropsychologia, 49, 3670–6.
- Deen B., Pitskel N.B., Pelphrey K.A. (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex, 21, 1498–506.
- Dimberg U., Thunberg M., Elmehed K. (2000). Unconscious facial reactions to emotional facial expressions. Psychological Science, 11, 86–9.
- Facchinetti L.D., Imbiriba L.A., Azevedo T.M., Vargas C.D., Volchan E. (2006). Postural modulation induced by pictures depicting prosocial or dangerous contexts. Neuroscience Letters 410, 52–6.
- Fadiga L., Craighero L., Buccino G., Rizzolatti G. (2002). Speech listening specifically modulates the excitability of tongue muscles: A TMS study. European Journal of Neuroscience, 15, 399–402.
- Fadiga L., Craighero L., Olivier E. (2005). Human motor cortex excitability during the perception of others' action. Current Opinion in Neurobiology, 15, 213–8.
- Farina S., Valeriani M., Rosso T., . (2001). Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neuroscience Letters, 314, 97–101.
- Fino E., Menegatti M., Avenanti A., Rubini M. (2016). Enjoying vs. smiling: Facial muscular activation in response to emotional language. Biological Psychology, 118, 126–35.
- Frijda N.H. (1986). The Emotions. Cambridge: Cambrigde University Press.
- Frijda N.H. (2009). Emotion experience and its varieties. Emotion Reviews, 1, 264–71.
- Gallese V., Sinigaglia C. (2011). What is so special about embodied simulation? Trends in Cognitive Science, 15, 512–9.
- Hagenaars M.A., Oitzl M., Roelofs K. (2014). Updating freeze: aligning animal and human research. Neuroscience Biobehavioral Review, 47, 165–76.
- Haidt J., McCauley C., Rozin P. (1994). Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual Differences, 16, 701–13.
- Hess U., Fisher A. (2014). Emotional mimicry: why and when we mimic emotions. Social and Personality Psychology Compass, 8, 45–57.
- Ibañez A., Gleichgerrcht E., Manes F. (2010). Clinical effects of insular damage in humans. Brain Structure and Function, 214, 397–410.
- Jabbi M., Bastiaansen J., Keysers C. (2008a). A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One, 3, e2939.
- Jabbi M., Keysers C. (2008b). Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion, 8, 775–80.
- Jabbi M., Swart M., Keysers C. (2007). Empathy for positive and negative emotions in the gustatory cortex. Neuroimage, 34, 1744–53.
- Jacquet P.O., Avenanti A. (2015). Perturbing the action observation network during perception and categorization of actions’ goals and grips: state-dependency and virtual lesion TMS effects. Cerebral Cortex, 25, 598–608.
- Jezzini A., Caruana F., Stoianov I., Gallese V., Rizzolatti G. (2012). Functional organization of the insula and inner perisylvian regions. Proceedings of the National Academy of Sciences of the United States of America, 109, 10077–82.
- Keysers C., Gazzola V. (2009). Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current Opinion in Neurobiology, 19, 666–71.
- Koch G., Versace V., Bonnì S., . (2010). Resonance of cortico-cortical connections of the motor system with the observation of goal directed grasping movements. Neuropsychologia, 48, 3513–20.
- Komeilipoor N., Vicario C.M., Daffertshofer A., Cesari P. (2014). Talking hands: tongue motor excitability during observation of hand gestures associated with words. Frontiers in Human Neuroscience, 8, 767.
- Krolak-Salmon P., Hénaff M.A., Isnard J., . (2003). Attention modulated response to disgust in human ventral anterior insula. Annual Neurology, 53, 446–53.
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54, 2492–502.
- Lamm C., Majdandžić J. (2015). The role of shared neural activations, mirror neurons, and morality in empathy–a critical comment. Neuroscience Research, 90, 15–24.
- Lamm C., Silani G., Singer T. (2015). Distinct neural networks underlying empathy for pleasant and unpleasant touch. Cortex, 70, 79–89.
- Lang P.J., Bradley M.M. (2010). Emotion and the motivational brain. Biological Psychology, 84, 437–50.
- Magnée M.J., Stekelenburg J.J., Kemner C., de Gelder B. (2007). Similar facial electromyographic responses to faces, voices, and body expressions. Neuroreport, 18, 369–72.
- Makin T.R., Holmes N.P., Brozzoli C., Rossetti Y., Farnè A. (2009). Coding of visual space during motor preparation: approaching objects rapidly modulate corticospinal excitability in hand-centered coordinates. Journal of Neuroscience, 29, 11841–51.
- Matsuo K., Palmer J.B. (2008). Anatomy and physiology of feeding and swallowing – normal and abnormal. Physical Medicine and Rehabilitation Clinics of North America, 19(4), 691–707.
- Meister I.G., Boroojerdi B., Foltys H., Sparing R., Huber W., Töpper R. (2003). Motor cortex hand area and speech: implications for the development of language. Neuropsychologia, 41, 401–6.
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214, 655–67.
- Mesulam M.M., Mufson E.J. (1982). Insula of the old world monkey. III: efferent cortical output and comments on function. Journal of Comparative Neurology, 212, 38–52.
- Minio-Paluello I., Baron-Cohen S., Avenanti A., Walsh V., Aglioti S.M. (2009). Absence of embodied empathy during pain observation in Asperger syndrome. Biological Psychiatry, 65, 55–62.
- Mistry S., Verin E., Singh S., . (2007). Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. Journal of Physiology, 585, 525–38.
- Muellbacher W., Mamoli B. (1997). The course of cortico-hypoglossal projections in the human brainstem: functional testing using transcranial magnetic stimulation. Brain, 120(10), 1909.
- Muellbacher W., Mathis J., Hess C.W. (1994). Electrophysiological assessment of central and peripheral motor routes to the lingual muscles. Journal of Neurology Neurosurgery and Psychiatry, 57, 309–15.
- Mukamel R., Ekstrom A., Kaplan J., Iacoboni M., Fried I. (2010). Single-neuron responses in humans during execution and observation of actions. Current Biology, 20, 750–6.
- Napadow V., Sheehan J.D., Kim J., . (2013). The brain circuitry underlying the temporal evolution of nausea in humans. Cerebral Cortex, 23, 806–13.
- Niedenthal P.M., Mermillod M., Maringer M., Hess U. (2010). The Simulation of Smiles (SIMS). model: embodied simulation and the meaning of facial expression. Behavioral and Brain Science, 33, 417–33.
- Oaten M., Stevenson R.J., Case T.I. (2009). Disgust as a disease-avoidance mechanism. Psychological Bulletin, 135, 303–21.
- Oberman L.M., Ramachandran V.S. (2007). The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin, 133, 310–27.
- O'Doherty J., Rolls E.T., Francis S., Bowtell R., McGlone F. (2001). Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology, 85, 1315–21.
- Olatunji B.O., Williams N.L., Tolin D.F., . (2007). The Disgust Scale: item analysis, factor structure, and suggestions for refinement. Psychological Assessment, 19, 281–97.
- Ostrowsky K., Isnard J., Ryvlin P., Guénot M., Fischer C., Mauguière F. (2000). Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia, 41, 681–6.
- Penfield W., Faulk M.E. Jr (1955). The insula; further observations on its function. Brain, 78, 445–70.
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety of TMS Consensus Group., (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–39.
- Rossini P.M., Burke D., Chen R., . (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology, 126, 1071–107.
- Rozin P., Fallon A.E. (1987). A perspective on disgust. Psychological Review, 94, 23–41.
- Rozin P., Haidt J., McCauley C. (2000). Disgust. In: Lewis M, Haviland M, editors. Handbook of Emotions, 637–53. UK: Guilford.
- Rütgen M., Seidel E.M., Riečanský I., Lamm C. (2015a). Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. Journal of Neuroscience, 35, 8938–47.
- Rütgen M., Seidel E.M., Silani G., Riečanský I., Hummer A., . (2015b). Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proceedings of the National Academy of Science of the United States of America, 112, E5638–46.
- Sato M., Buccino G., Gentilucci M., Cattaneo L. (2010). On the tip of the tongue: modulation of the primary motor cortex during audiovisual speech perception. Speech Communication, 52, 533–41.
- Schäfer A., Leutgeb V., Reishofer G., Ebner F., Schienle A. (2009). Propensity and sensitivity measures of fear and disgust are differentially related to emotion-specific brain activation. Neuroscience Letters, 465, 262–6.
- Schienle A., Schäfer A., Hermann A., Walter B., Stark R., Vaitl D. (2006). fMRI responses to pictures of mutilation and contamination. Neuroscience Letters 393, 174–8.
- Schienle A., Schäfer A., Walter B., Stark R., Vaitl D. (2005). Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neuroscience Letters, 388, 1–6.
- Schutter D.J.L.G., Hofman D., Van Honk J. (2008). Fearful faces selectively increase corticospinal motor tract excitability: a transcranial magnetic stimulation study. Psychophysiology, 45, 345–8.
- Selimbeyoglu A., Parvizi J. (2010). Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience, 4, 46.
- Serino A., Annella L., Avenanti A. (2009). Motor properties of peripersonal space in humans. PLoS One, 4, e6582.
- Serino A., Canzoneri E., Avenanti A. (2011). Fronto-parietal areas necessary for a multisensory representation of peripersonal space in humans: an rTMS study. Journal of Cognitive Neuroscience, 23, 2956–67.
- Steele C.M., Miller A.J. (2010). Sensory input pathways and mechanisms in swallowing: a review. Dysphagia, 25, 323–33.
- Terasawa Y., Kurosaki Y., Ibata Y., Moriguchi Y., Umeda S. (2015). Attenuated sensitivity to the emotions of others by insular lesion. Frontiers in Psychology, 6, 1314.
- Tidoni E., Borgomaneri S., di Pellegrino G., Avenanti A. (2013). Action simulation plays a critical role in deceptive action recognition. Journal of Neuroscience, 33, 611–23.
- Toki S., Okamoto Y., Onoda K., . (2013). Automatic and intentional brain responses during evaluation of face approachability?: correlations with trait anxiety. Neuropsychobiology, 68, 156–67.
- Tokimura H., Tokimura Y., Oliviero A., Asakura T., Rothwell J.C. (1996). Speech-induced changes in corticospinal excitability. Annals of Neurology, 40, 628–34.
- Tottenham N., Tanaka J.W., Leon A.C., . (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168, 242–9.
- Urban P.P., Hopf H.C., Connemann B., Hundemer H.P., Koehler J. (1996). The course of corticohypoglossal projections in the human brainstem. Functional testing using transcranial magnetic stimulation. Brain, 119, 1031–8.
- Urban P.P., Solinski M., Best C., Rolke R., Hopf H.C., Dieterich M. (2004). Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle Nerve, 29, 663–9.
- van der Gaag C., Minderaa R.B., Keysers C. (2007). Facial expressions: what the mirror neuron system can and cannot tell us. Social Neuroscience, 2, 179–222.
- van Loon A.M., van den Wildenberg W.P., van Stegeren A.H., Hajcak G., Ridderinkhof K.R. (2010). Emotional stimuli modulate readiness for action: a transcranial magnetic stimulation study. Cognitive Affective and Behavioral Neuroscience, 10, 174–81.
- Vicario C.M. (2014). Speech prosody, reward, and the corticobulbar system: an integrative perspective. Behavioral and Brain Science, 37, 573–4.
- Vicario C.M., Candidi M., Aglioti S.M. (2013a). Cortico-spinal embodiment of newly acquired, action-related semantic associations. Brain Stimulation, 6, 952–8.
- Vicario C.M., Komeilipoor N., Cesari P., Rafal R.D., Nitsche M.A. (2014). Enhanced corticobulbar excitability in chronic smokers during visual exposure to cigarette smoking cues. The Journal of Psychiatry and Neuroscience, 39, 232–8.
- Vicario C.M., Kritikos A., Avenanti A., Rafal R. (2013b). Reward and punishment: investigating cortico-bulbar excitability to disclose the value of goods. Frontiers in Psychology, 4, 39.
- Vicario C.M., Rafal R.D., Avenanti A. (2015). Counterfactual thinking affects the excitability of the motor cortex. Cortex 65, 139–48.
- Vicario C.M., Newman A. (2013). Emotions affect the recognition of hand gestures. Frontiers in Human Neuroscience, 7, 906.
- Wicker B., Keysers C., Plailly J., Royet J.P., Gallese V., Rizzolatti G. (2003). Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron, 40, 655–64.
- Zaki J., Wager T.D., Singer T., Keysers C., Gazzola V. (2016). The anatomy of suffering: understanding the relationship between nociceptive and empathic pain. Trends in Cognitive Science, 20, 249–59.
