CRISPR technology: A decade of genome editing is only the beginning
- Wang, Joy Y.
- Doudna, Jennifer A.
A decade of CRISPR
In the decade since the publication of CRISPR-Cas9 as a genome-editing technology, the CRISPR toolbox and its applications have profoundly changed basic and applied biological research. Wang and Doudna now review the origins and utility of CRISPR-based genome editing, the successes and current limitations of the technology, and where innovation and engineering are needed. The authors describe important advances in the development of CRISPR genome-editing technology and make predictions about where the field is headed. They also highlight specific examples in medicine and agriculture that show how CRISPR is already affecting society, with exciting opportunities for the future. —DJ
BACKGROUND:
The fields of molecular biology, genetics, and genomics are at a critical juncture—a moment in history when a convergence of knowledge and methods has made it both technically possible and incredibly useful to edit specific base pairs or segments of DNA in cells and living organisms. The advent of clustered regularly interspaced short palindromic repeat (CRISPR) genome editing, coupled with advances in computing and imaging capabilities, has initiated a new era in which we can not only diagnose human diseases and even predict individual susceptibility based on personal genetics but also act on that information. Likewise, we can both identify and rapidly alter genes responsible for plant traits, transforming the pace of agricultural research and plant breeding. The applications of this technology convergence are profound and far reaching—and they are happening now. In the decade since the publication of CRISPR-Cas9 as a genome editing technology, the CRISPR toolbox and its applications have profoundly changed biological research, impacting not only patients with genetic diseases but also agricultural practices and products. As a specific example from the field of genomic medicine, it has become feasible to obtain a complete sequence of the human genome in less than 24 hours—a staggering advance considering the first such sequence took 5 years to generate. Notably, designing and putting to use a potent CRISPR genome editor to obtain clinically actionable information from that genome—previously a near-intractable challenge—now takes only a matter of days. For additional background and related topics, we refer readers to in-depth reviews of the microbiology and structural biology of CRISPR systems and to articles about the considerable ethical and societal challenges of this technology.
ADVANCES:
The past decade has witnessed the discovery, engineering, and deployment of RNA-programmed genome editors across many applications. By leveraging CRISPR-Cas9's most fundamental activity to create a targeted genetic disruption in a gene or gene regulatory element, scientists have built successful platforms for the rapid creation of knockout mice and other animal models, genetic screening, and multiplexed editing. Beyond traditional CRISPR-Cas9-induced knockouts, base editing—a technology utilizing engineered Cas9's fused to enzymes that alter the chemical nature of DNA bases—has also provided a highly useful strategy to generate site-specific and precise point mutations. Over the past decade, scientists have utilized CRISPR technology as a readily adaptable tool to probe biological function, dissect genetic interactions, and inform strategies to combat human diseases and engineer crops. This Review covers the origins and successes of CRISPR-based genome editing and discusses the most pressing challenges, which include improving editing accuracy and precision, implementing strategies for precise programmable genetic sequence insertions, improving targeted delivery of CRISPR editors, and increasing access and affordability. We examine current efforts addressing these challenges, including emerging gene insertion technologies and new delivery modalities, and describe where further innovation and engineering are needed. CRISPR genome editors are already being deployed in medicine and agriculture, and this Review highlights key examples, including a CRISPR-based therapy treating sickle cell disease, a more nutritious CRISPR-edited tomato, and a high-yield, disease-resistant CRISPR-edited wheat, to illustrate CRISPR's current and potential future impacts in society.
OUTLOOK:
In the decade ahead, genome editing research and applications will continue to expand and will intersect with advances in technologies, such as machine learning, live cell imaging, and sequencing. A combination of discovery and engineering will diversify and refine the CRISPR toolbox to combat current challenges and enable more wide-ranging applications in both fundamental and applied research. Just as during the advent of CRISPR genome editing, a combination of scientific curiosity and the desire to benefit society will drive the next decade of innovation in CRISPR technology.
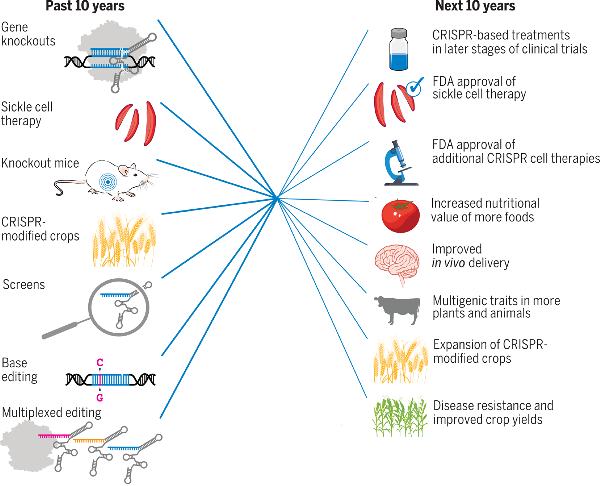
Figure
CRISPR: past, present, and future. The past decade of CRISPR technology has focused on building the platforms for generating gene knockouts, creating knockout mice and other animal models, genetic screening, and multiplexed editing. CRISPR's applications in medicine and agriculture are already beginning and will serve as the focus for the next decade as society's demands drive further innovation in CRISPR technology.
The advent of clustered regularly interspaced short palindromic repeat (CRISPR) genome editing, coupled with advances in computing and imaging capabilities, has initiated a new era in which genetic diseases and individual disease susceptibilities are both predictable and actionable. Likewise, genes responsible for plant traits can be identified and altered quickly, transforming the pace of agricultural research and plant breeding. In this Review, we discuss the current state of CRISPR-mediated genetic manipulation in human cells, animals, and plants along with relevant successes and challenges and present a roadmap for the future of this technology.
