Significance Statement
Despite the current standard of care of hypothermia (HT), newborns with HIE suffer increased mortality and neurological damage impacting normal growth and development. This report describes a phase I study of intravenously infused allogeneic hCT-MSC combined with HT. Infusions were well tolerated and no acute toxicity or serious adverse events were noted in the 12 months of follow-up. All the babies survived and all developmental assessment standard scores obtained between 12 and 17 months were within the average to low-average range for age. Further testing is needed, but availability of an off-the-shelf cellular therapy for HIE could improve outcomes for these babies.
Introduction
Treatment of term and near-term infants with neonatal encephalopathy and clinical findings suggestive of hypoxic-ischemic injury (hypoxic-ischemic encephalopathy, HIE) with hypothermia for 72 h reduces risk of death or survival with neurodevelopmental impairment in high resource settings. While outcomes for infants treated with hypothermia are improved compared to those managed without hypothermia, a considerable portion of patients enrolled in clinical trials and treated with therapeutic hypothermia either, died or survived with impairment, leading to ongoing efforts to establish safety and efficacy for adjunct therapies. Pre-clinical and early clinical trial evidence suggest that cell-based therapies, working via paracrine mechanisms, may provide benefits to infants with HIE. In our double-blind, randomized clinical trial of autologous cord blood cells for infants treated with hypothermia with HIE, we found that implementing logistics for cord blood collection, processing and infusion in the first postnatal hours was a significant barrier. Thus, an off-the-shelf, safe and effective product would provide significant advantages.
Mesenchymal stromal cells (MSCs) are a heterogeneous group of multipotent cells that can be isolated from several different tissues including bone marrow, adipose tissue, and fetal tissues (umbilical cord blood, umbilical cord tissue, amniotic fluid, and placenta). Use of mesenchymal stromal cells (MSCs) from a number of sources, including cord tissue, resulted in anatomic sparing of brain tissue, enhanced neurotrophic factor levels, and improved neurological outcomes in animal models of neonatal HIE. In an in vitro model utilizing a purified rat retinal ganglion cell culture system, cord tissue derived MSCs (hCT-MSCs) secreted factors that strongly promote excitatory synaptic connectivity and enhance neuronal survival. Safety of administration of MSCs has been reported in dozens of clinical trials testing MSCs from multiple sources in over 1000 study participants, including hundreds of children, with cardiovascular, neurological, oncologic, metabolic, gastrointestinal, and post-transplant conditions, such as steroid-resistant graft versus host disease. In preterm neonates, allogeneic MSCs derived from healthy donor cord blood have been tested in phases 1 and 2 clinical trials targeting the treatment of evolving lung disease as well as post-hemorrhagic hydrocephalus.
With emerging preclinical data suggesting positive effects after hypoxic-ischemic brain injury and safety data for use of MSCs in multiple clinical trials inclusive of children, as well as acknowledging the challenges in feasibility of collecting, processing, and infusing autologous cord blood cells in the first postnatal hours, we conducted a phase I, open-label clinical trial of intravenously administered umbilical cord tissue-derived allogeneic MSCs for term and near-term infants who met criteria for therapeutic hypothermia for moderate to severe HIE.
Material and Methods
Study Population
Infants admitted to the Duke Neonatal Intensive Care Unit (NICU) were eligible if they were ≥ 35 weeks gestation with HIE and met the Neonatal Intensive Care Unit’ (NICU)’s cooling criteria, which is based on the inclusion criteria used in the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Hypothermia trial., Hypothermia criteria were met if infants had cord or first postnatal hour blood gas results with pH ≤ 7.0, or base deficit ≥−16. If a blood gas in the first postnatal hour was unavailable, or if the cord or first postnatal hour blood gas pH was 7.01-7.15 or base deficit between −10 and −15, infants were eligible if they also had a history of an acute perinatal event and either an Apgar score at 10 min of ≤5 or need for positive pressure ventilation initiated at birth and continued for ≥10 min. Infants meeting the criteria were then examined in 6 domains: level of consciousness, level of spontaneous activity, tone, posture, primitive reflexes, and autonomic function. If abnormal in 3 of 6 domains, or if they had seizures, they were treated with hypothermia and eligible for the study. Study staff approached parents of eligible infants on the first postnatal day, with a goal of obtaining consent in the first 2 postnatal days to allow for dosing of enrolled infants in the first 48 postnatal hours.
Umbilical Cord Tissue-Derived MSCs (hCT-MSCs)
hCT-MSCs were manufactured from umbilical cord tissue donated to the Carolinas Cord Blood Bank, an FDA-licensed, FACT-accredited, public cord blood bank at Duke University Medical Center, after written informed consent from the donor baby’s mother. For cell preparation of hCT-MSCs, cord tissue is harvested from the placentas of male babies delivered by elective C-section after a normal, full-term pregnancy. The use of male donors allows for the assessment of the purity of the final product through screening for contaminating maternal cells. Donor screening questionnaires are completed by the maternal donor, and maternal blood is tested for relevant communicable diseases per CFR 1271.75. After delivery of the placenta and cord, the cord blood is aseptically drained from the placenta. Then the cord is dried and cleaned with ChloraPreps (BD, Franklin Hills, NJ, USA), separated from the base of the placenta, placed in a sterile bottle containing plasmalyte-A (Baxter Healthcare, Deerfield, IL), and transported at room temperature in a validated container to the Marcus Center for Cellular Cures (MC3) Robertson GMP cell processing laboratory. In the clean room manufacturing suite, using a biosafety cabinet, the cord tissue is removed from the media, placed in sterile dishes, cut into small pieces, and then minced and digested in the Miltenyi Biotec GentleMacs Octo Dissociator (Miltenyi Biotec, North Rhine-Wesphalia, Germany) with proprietary concentrations of GMP-grade enzymes: hyaluronidase, DNase, collagenase, papain. The resultant cell suspension is cultured in Prime XV MSC Expansion XSFM (FUJIFILM Irvine Scientific, Santa Ana, CA) media with 1% platelet lysate (Compass Biomedical, Cleveland, OH) and grown to confluence (~7-14 days) to establish the P0 culture. To establish the master cell bank, P0 is harvested and cryopreserved in cryovials with Cryostor 10 media (BioLife Solutions, Bothell, WA), and stored in the vapor phase of liquid nitrogen. P1 and P2 cultures are grown under similar conditions, in hyperflasks or hyperstacks (Corning Life Sciences, Corning, NY) without platelet lysate, as needed to create the working cell bank and product for administration, respectively. Cells from P1 and P2 are removed from plastic cultureware using TrypLE (Gibco ThermoFisher Scientific, https://www.thermofisher.com/us/en/home.html). The final product is derived from the P2 cultures which are harvested into plasmalyte-A with 5% human serum albumin, washed, and cryopreserved in 5 compartment cryobags (Syngen Inc, New York, NY) in 5 mL containing 50-100 million cells in a final concentration of 10% DMSO with dextran (Akron Biotech, Sarasota, FL). At each passage, the cell product is characterized by assessing yields, viability, sterility, and cell surface phenotype by flow cytometry. Each lot, prior to cryopreservation of P2, is also tested for sterility, endotoxin, and mycoplasma and these tests must meet specifications. A representative cryopreserved sample from each lot is thawed and tested for cell recovery, viability, phenotype, endotoxin, mycoplasma, sterility, adventitial viruses, ability to differentiate into fat, cartilage, and bone, and potency tested by suppression of 3rd party T-cell proliferation.
One lot of hCT-MSCs was selected for this clinical trial. For dosing, release testing includes total nucleated cell count (TNCC), viability, gram stain, and endotoxin. On the day of administration, one compartment is thawed, diluted 1:1 in Plasmalyte-Z + 5% human serum albumin (HAS). A small sample is removed and counted and tested for viability on a Cellometer (Nexcelom Bioscience, Lawrence, MA). Viability must be ≥70% to proceed to administration. The volume calculated to deliver 2 × 106 cells/kg is calculated and further diluted in 6–9 mL of plasmalyte-A + 5% HSA IV solution, placed in a syringe and transported to the bedside for administration over 30-60 min. We aimed to enroll 6 infants. The first 3 enrolled infants would receive a single dose of hCT-MSCs in the first 48 postnatal hours, and the second 3 infants would receive a second dose at 2 months of age in addition to the first dose during hypothermia. Each dose consisted of 2 × 106 hCT-MSCs/kg.
Intervention/Infusion
All infants were cooled to 33.5 °C for 72 h following the NICU’s usual practice parameter based on the published protocol., Study staff carried pre-measured doses of cells to the NICU in labeled syringes from the Duke Stem Cell Laboratory. Infusions were started when cells and study staff were available for administration and monitoring. Infants were pretreated with hydrocortisone, 1 mg/kg IV 30-60 min prior to infusion. Infusate and subject identities were double-checked by research and clinical nursing staff. Infusions were monitored by research and clinical staff. Cells were infused over 30 min, followed by a 1- to 2-mL saline flush to clear the intravascular line.
Statistical Analyses
We used descriptive statistics to characterize subject demographics. We compared vital signs pre- and post the first and second infusions and monitored adverse events with a primary focus on infusion-related infection and infusion reactions.
Outcome Assessments
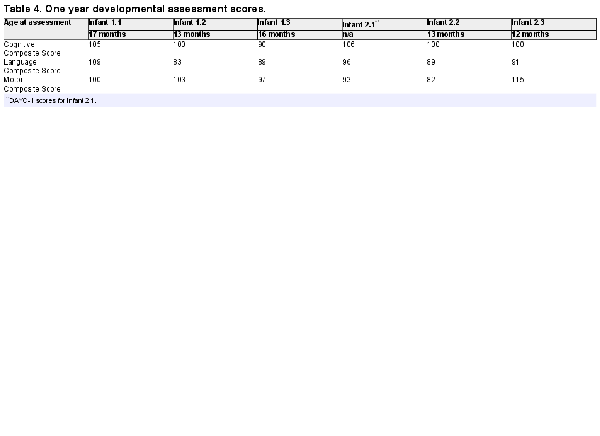
One-year neurodevelopmental outcomes were assessed utilizing the Bayley Scales of Infant and Toddler Development-3rd Edition (Bayley III) which includes assessment of cognitive, language, and motor developmental domains (scale mean = 100, SD = 15). Bayley-III assessments were completed by a doctoral level pediatric psychologist research reliability certified in Bayley-III administration. In addition, we monitored panel-reactive antibodies at 2 months, and 1-year post infusion. Panel reactive antibodies (PRAs) provide an estimate of an individual’s antibody response to specific HLA antibodies.
The study was approved by the Duke IRB, listed on clinical trials.gov (NCT03635450), and carried out under FDA IND 17313.
Results
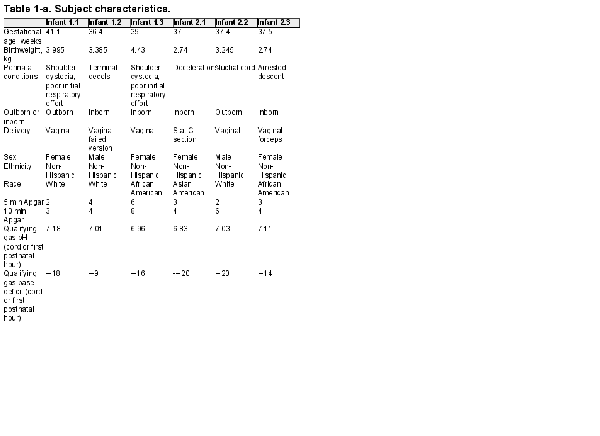
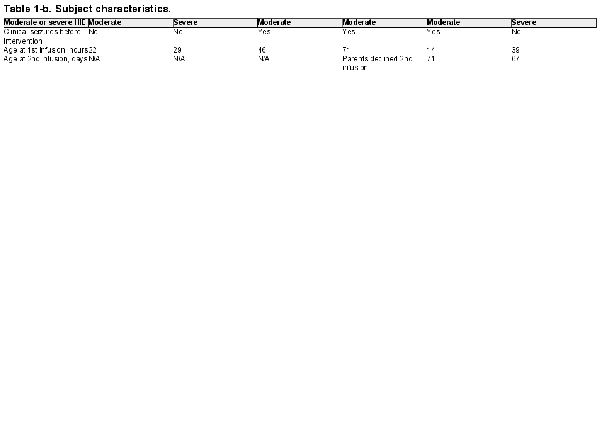
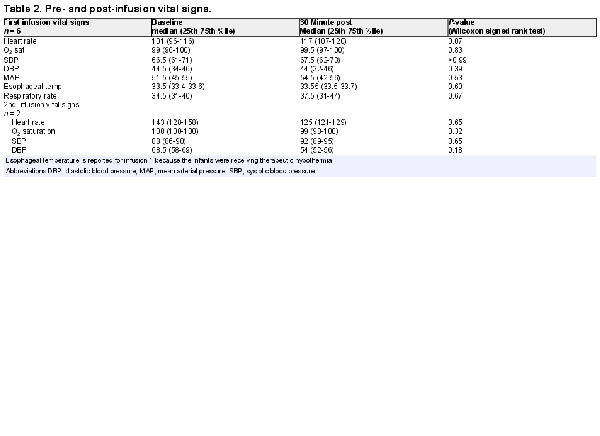
Infants were screened for enrollment beginning in March 2019. By August 1, 2019, 18 infants with moderate to severe HIE were admitted to the Duke NICU and treated for hypothermia. Four of these infants failed screening and the families of 14 infants were approached to request consent for their infants to participate in this study (Figure 1). Six families agreed to enroll. Characteristics of the enrolled infants are listed in Table 1. Of note, 4 infants were assessed before hypothermia with moderate encephalopathy, and 2 infants were classified as having severe encephalopathy. Two infants were outborn and transferred to our facility within 4 postnatal hours. While the target for the first infusion was the first 48 postnatal hours, 1 infant received the first infusion at 71 postnatal hours. This baby was among the second 3, with a planned second infusion; however, the family declined the second infusion. Pre- and post-infusion median and 25th and 75th percentiles for vital signs, for the first and second infusions, are provided in Table 2. No statistically significant differences were observed.
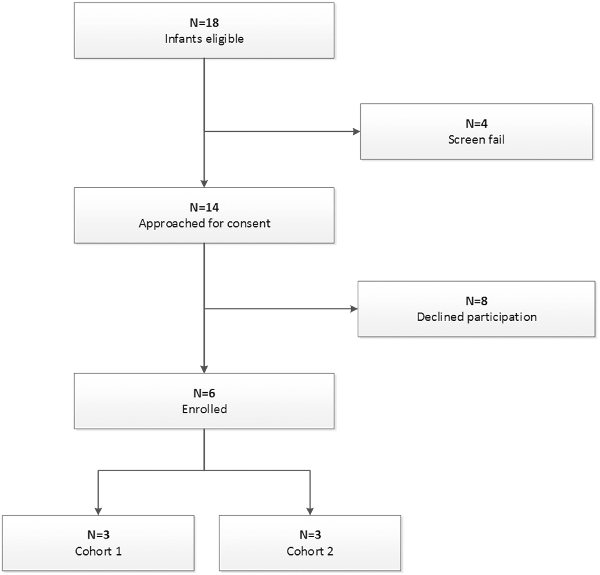
Figure 1
CONSORT diagram of screened and enrolled infants.
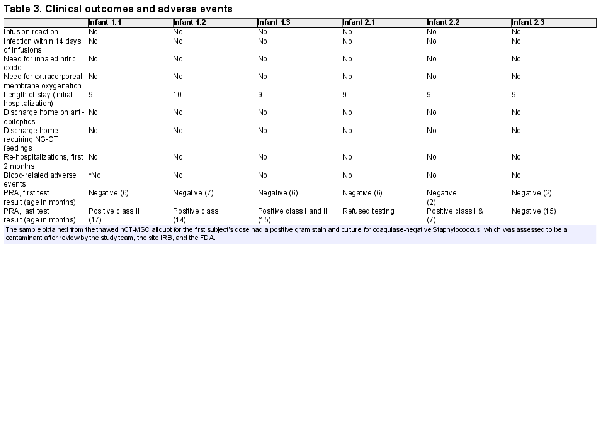
In-hospital and post-discharge outcomes are provided in Table 3. Of note, none of the readings of the MRI’s obtained on the 6 infants at postnatal day 5 had findings consistent with hypoxic-ischemic injury. Two adverse events were reported. The first incident occurred with the first enrolled infant. Nineteen hours after the infusion, the study staff was notified by the stem cell lab that the broth culture taken from the thawed hCT-MSC cell product as part of Quality Control (QC) procedures during dose preparation had been signaled as positive. The culture obtained from the cell preparation prior to freezing had been sterile. A Gram stain from this positive broth culture indicated “Gram positive cocci in clusters” and the infant’s clinical team and the infant’s parents were informed. A second clinical blood culture was obtained and the infant was empirically started on vancomycin. The bacteria from the broth culture was identified as Staphylococcus epidermidis (coagulase negative) 2 days later. The infant’s blood culture remained negative, and the infant completed 48 h of vancomycin with no sign of infection. Of note, no other positive cultures were noted throughout the course of the study. The infant had re-warmed without incident and appeared well, and the baby’s initial clinical blood culture was negative. The incident was reported to the Duke IRB as well as the FDA. The final determination was that it was highly likely that the broth culture was contaminated during handling post-thaw, rather than cell product contamination, so study enrollment was allowed to continue. Of note, the hCT-MSC cell product undergoes extensive QC testing during production and prior to infusion. It is stored frozen and is thawed in aliquots as needed for infusion. Other aliquots from this lot of hCT-MSCs have been thawed and have had negative gram stains and cultures. The test thaw from the GMP lab’s manufacturing lot was also negative. The second reported adverse event was one bloody stool in one infant occurring post-discharge home, likely due to milk protein allergy. It resolved without incident.
In follow-up safety laboratory testing for panel reactive antibodies, 5 of 6 infants eventually tested positive. None of the 6 infants received red blood cell or platelet transfusions. While expected, and not considered an adverse event in the trial, we consider this important to report as the presence of the antibodies may impact compatibility with cell products that may be used for these babies later in life.
Bayley-III cognitive, language, and motor composite scores are listed in Table 4. For the 5 infants who did return for neurodevelopmental follow-up, the age at assessment ranged from 12 to 17 months. Citing reluctance to go to study-only related clinic visits during the COVID pandemic, 1 family did not return to the clinic for the second infusion and blood draws, and the in-person neurological assessment. As the Bayley-III cannot be completed via telehealth, for this infant, a telehealth assessment was completed at 12 months of age using the Developmental Assessment of Young Children-II (DAYC-II), which is highly correlated with the Bayley-III. All developmental assessment standard scores were within the average to low average range for age.
Discussion
To the best of our knowledge, we are reporting results from the first clinical trial to test the safety and feasibility of intravenous administration of allogeneic cord—tissue-derived MSCs for moderate to severe hypoxic-ischemic encephalopathy in term and near-term infants. In this study, 1 or 2 intravenous 2 × 106 cells/kg doses of allogeneic human umbilical cord tissue-derived MSCs were not associated with immediate SAEs in our small cohort of term and near-term born infants. These findings suggest that intravenous dosing of hCT-MSCs in term and near-term infants with moderate to severe HIE may be safe and feasible.
The only potential safety issue identified in this small cohort was the development of anti-class I HLA antibodies in the majority of participants. We observed this phenomenon in previous studies of hCT-MSC in older children with autism spectrum disorder or cerebral palsy (add citations) without clinical sequelae to date., These antibodies, if persistent, could impact selection of donors for transplantation should the baby have a need for transplantation therapy in their lifetime. Of note, the development of anti-HLA antibodies could also occur after a red blood cell or platelet transfusion if administered as part of standard medical care. Thus, this risk is not restricted to the use of MSC as a cell therapy product.
Investigators continue to test cell-based therapies in pre-clinical animal models, but few have included both cell therapy and hypothermia. The first report, from Park et al., tested human cord blood-derived MSCs in a P7 rat model of hypoxic-ischemic brain injury administered immediately before cooling. The combination of cells followed by cooling resulted in greater improvement than with either therapy alone. This report was followed by a report from Herz et al., in which murine bone marrow derived MSCs were administered intranasally 3 days after injury and cooling. In that study, anatomic and functional outcomes for mice treated with both cooling and cells were not as good as results for animals treated with cells or hypothermia alone. In vitro coculture experiments with brain slices from cooled animals and non-cooled animals found increased MSC gene expression and production of inflammatory cytokines and decreased neurotrophic factors in the cells cocultured with brain slices obtained from animals that were cooled. This report was followed by another report from Park’s group, testing human cord blood-derived MSCs administered after cooling in rat pups with hypoxic-ischemic injury on P7. In this study, as in this group’s prior report, the combination of cooling plus cells resulted in less anatomic injury and better functional outcomes. More recently, in a large animal model, 17 newborn piglets with hypoxic-ischemic brain injury were treated with 12 h of hypothermia alone (n = 7) or hypothermia plus two 30 × 106 doses of single umbilical cord donor-derived MSCs, either intranasally (n = 5) or intravenously (n = 5) at 24 and 48 h. In this short-term outcome study, which included MR-spectroscopy and cell-level effects, intranasal and intravenous administration of MSCs after injury and cooling was safe, and intranasal administration of a higher dose of cells than the dose we used, plus cooling, provided a modest benefit.
While our study suggests that intravenous administration of an allogeneic, off-the-shelf MSC product in newborn infants with HIE is feasible, uncertainties remain about the best source of cells. We are re-assured by in vivo evidence suggesting MSCs derived from umbilical cord tissue, or other “fetal” derived sources such as cord blood-derived MSCs, may provide higher likelihood of benefit compared with MSCs derived from adult tissue. Hsieh et al. reported studies comparing cord tissue-derived MSCs with adult marrow-derived MSCs. Cord tissue-derived MSCs expressed more angiogenesis and neurogenesis factor genes, and in cell culture, induced neural cell differentiation and migration than marrow-derived MSCs. In an oxygen-glucose deprivation injury cell-culture model, the cord derived MSCs led to improved survival of neural cells. More specifically in peripheral nerve injury repair, studies of cord-tissue-derived MSCs identified 14 cytokines and neurotrophic factors produced by the cord-tissue-derived MSCs, re-iterating the likely paracrine mechanisms underlying effects noted so far in animal models of injury.
Another area of uncertainty is how intravenously administered MSCs, most of which are delivered to the lung, exert effects in distal injured organs like the brain. Recently Min et al. reported that after IV administration, MSCs interact with lung macrophages exchanging bits of RNA through cytoplasmic RNA processing bodies (P-bodies). The P-bodies trigger secondary signaling through factors secreted by the lung macrophages to multiple organs including the brain.
While a systematic review of the studies of use of MSCs for neonatal brain injury models shows anatomic and functional outcome benefits for cell recipients, the review clearly demonstrates variation in dose amounts, dosing strategies, and measures of outcomes. A more recent study of administration of cord tissue-derived MSCs administered in P10 rat pups with hypoxic-ischemic brain injury model, cell recipients had improved anatomic and functional outcomes, as well as decreased caspase-3 and Beclin-2 in injured tissue. In addition to dosing and delivery variations in the studies included in the review, the study by Herz found better outcome with cells alone or cooling alone than the combination, the study used rodent-marrow derived cells, and not cord tissue or cord blood derived MSCs. The studies by Park et al., which included hypothermia plus administration of MSCs, before or after cooling, found some benefits to the combination therapy, although the Robertson study did not include a “cells alone” group.,, These results, plus the accumulating safety data from multiple pediatric populations, encouraged us to move forward with the phase I study to test feasibility and safety. Preclinical studies will continue to inform design of subsequent clinical trials.
Our study had several limitations. We elected to use a dose-per-infusion that had been previously used in studies of graft versus host disease (2 × 106 cells/kg twice weekly for 4 weeks), and was similar to the single dose used in the large animal study by Jellema., Because of the potential exposure to residual DMSO in the cell product which could cause hypersensitivity reactions in up to 5% of recipients, we included a dose of hydrocortisone prior to administration of cells, as we did in our prior phase I trial of autologous volume and red blood cell reduced cord blood mononuclear cells. We did not note adverse events related to hydrocortisone, or to the MSCs, but in future studies testing efficacy, the impact of the hydrocortisone dosing will have to be considered. We assessed neurodevelopment at 12-17 months and results were promising, with developmental functioning within the average to low average range for all children; however, longer term neurodevelopmental follow up is necessary to better understand outcomes in children obtaining this MSCs combined with hypothermia for neonatal HIE, particularly given the relatively low predictive utility of the Bayley-III for longer term developmental outcomes.
Conclusion
We completed a phase I open-label trial of allogeneic MSCs obtained from a healthy donor’s cord tissue and demonstrated feasibility and safety of infusion of cells during and 2 months after cooling. Further study, of dose amount, dose timing, in a randomized trial, inclusive of long-term follow-up for safety and neurodevelopmental outcome is warranted.
Acknowledgments
We thank the staff of the Robertson GMP Cell Manufacturing Laboratory for manufacturing and qualifying the hCT-MSCs, the staff of the Duke Stem Cell Transplant Laboratory for thawing and preparing the cells for administration, the nursing staff of the NICU for administering the cells and observing the patients post infusion, and the Marcus Foundation for supporting the costs of manufacturing the cells for this clinical trial. Research support: Duke Clinical and Translational Science Institute (CTSI), Marcus Foundation (manufacturing).
References
- 1. Shankaran S, Laptook AR, Ehrenkranz RA, et al; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. https://doi.org/10.1056/NEJMcps050929
- 2. Azzopardi DV, Strohm B, Edwards AD, et al; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. https://doi.org/10.1056/NEJMoa0900854
- 3. Jacobs SE, Morley CJ, Inder TE, et al; Infant Cooling Evaluation Collaboration. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700. https://doi.org/10.1001/archpediatrics.2011.43
- 4. Simbruner G, Mittal RA, Rohlmann F, Muche R; neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126(4):e771–e778. https://doi.org/10.1542/peds.2009-2441
- 5. Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2016;15(2):145–153. https://doi.org/10.1016/S1474-4422(15)00347-6
- 6. Juul SE, Comstock BA, Heagerty PJ, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): a randomized controlled trial - background, aims, and study protocol. Neonatology. 2018;113(4):331–338. https://doi.org/10.1159/000486820
- 7. Maiwald CA, Annink KV, Rüdiger M, et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). BMC Pediatr. 2019;19(1):210. https://doi.org/10.1186/s12887-019-1566-8
- 8. Bruschettini M, Romantsik O, Moreira A, Ley D, Thébaud B. Stem cell-based interventions for the prevention of morbidity and mortality following hypoxic-ischaemic encephalopathy in newborn infants. Cochrane Database Syst Rev. 2020;8(8):CD013202. https://doi.org/10.1002/14651858.CD013202.pub2
- 9. A Multi-site Study of Autologous Cord Blood Cells for Hypoxic Ischemic Encephalopathy. Clinicaltrials.gov. Published November 23, 2015. Updated August 25, 2020. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT02612155
- 10. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274.
- 11. Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. https://doi.org/10.1182/blood.v98.8.2396
- 12. Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–1337. https://doi.org/10.1634/stemcells.2004-0013
- 13. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. https://doi.org/10.1080/14653240600855905
- 14. Zhang X, Zhang Q, Li W, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res. 2014;92(1):35–45. https://doi.org/10.1002/jnr.23304
- 15. Jellema RK, Wolfs TG, Lima Passos V, et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. 2013;8(8):e73031. https://doi.org/10.1371/journal.pone.0073031
- 16. Donega V, Nijboer CH, van Velthoven CT, et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015;78(5):520–526. https://doi.org/10.1038/pr.2015.145
- 17. Archambault J, Moreira A, McDaniel D, et al. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: a systematic review and meta-analysis of preclinical studies. PLoS One. 2017;12(12):e0189895. https://doi.org/10.1371/journal.pone.0189895
- 18. Ahn SY, Chang YS, Sung DK, Sung SI, Park WS. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci Rep. 2018;8(1):7665. https://doi.org/10.1038/s41598-018-25902-x
- 19. Koh S, Kim N, Yin HH, et al. Human umbilical tissue-derived cells promote synapse formation and neurite outgrowth via thrombospondin family proteins. J Neurosci. 2015;35(47):15649–15665. https://doi.org/10.1523/JNEUROSCI.1364-15.2015
- 20. Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. https://doi.org/10.1371/journal.pone.0047559
- 21. Fisher SA, Cutler A, Doree C, et al. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst Rev. 2019;1(1):CD009768. https://doi.org/10.1002/14651858.CD009768.pub2
- 22. Thompson M, Mei SHJ, Wolfe D, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249. https://doi.org/10.1016/j.eclinm.2019.100249
- 23. Kurtzberg J, Abdel-Azim H, Carpenter P, et al; MSB-GVHD001/002 Study Group. A phase 3, single-arm, prospective study of remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26(5):845–854. https://doi.org/10.1016/j.bbmt.2020.01.018
- 24. Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164(5):966–972.e6. https://doi.org/10.1016/j.jpeds.2013.12.011
- 25. Powell SB, Silvestri JM. Safety of intratracheal administration of human umbilical cord blood derived mesenchymal stromal cells in extremely low birth weight preterm infants. J Pediatr. 2019;210:209–213.e2. https://doi.org/10.1016/j.jpeds.2019.02.029
- 26. Nguyen LT, Trieu TTH, Bui HTH, et al. Allogeneic administration of human umbilical cord-derived mesenchymal stem/stromal cells for bronchopulmonary dysplasia: preliminary outcomes in four Vietnamese infants. J Transl Med. 2020;18(1):398. https://doi.org/10.1186/s12967-020-02568-6
- 27. Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose-escalation clinical trial. Stem Cells Transl Med. 2018;7(12):847–856. https://doi.org/10.1002/sctm.17-0219
- 28. Ahn SY, Chang YS, Lee MH, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: a randomized controlled phase II trial. Stem Cells Transl Med. 2021;10(8):1129–1137. https://doi.org/10.1002/sctm.20-0330
- 29. Shankaran S, Laptook AR, Pappas A, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy a randomized clinical trial. JAMA. 2014;312(24):2629–2639. https://doi.org/10.1001/jama.2014.16058
- 30. CFR – Code of Federal Regulations Title 21. Fda.gov. Updated March 29, 2022. Accessed June 7, 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1271.75
- 31. Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: The Psychological Corporation; 2006.
- 32. About CPRA. Hrsa.gov. Accessed June 8, 2022. https://optn.transplant.hrsa.gov/resources/allocation-calculators/about-cpra/
- 33. Voress JK, Maddox T. Developmental Assessment of Young Children–Second Edition (DAYC-2). Austin, TX: PRO-ED. 2013.
- 34. Sun JM, Dawson G, Franz L, et al. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. Stem Cells Transl Med. 20201146;9(10):1137–1146. https://doi.org/10.1002/sctm.19-0434
- 35. Sun JM, Case LE, McLaughlin C, et al. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: an open-label, randomized trial. Dev Med Child Neurol. 2022;64(12):1477–1486. https://doi.org/10.1111/dmcn.15325
- 36. Park WS, Sung SI, Ahn SY, et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10(3):e0120893. https://doi.org/10.1371/journal.pone.0120893
- 37. Herz J, Köster C, Reinboth BS, et al. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav Immun. 2018;70:118–130. https://doi.org/10.1016/j.bbi.2018.02.006
- 38. Robertson NJ, Meehan C, Martinello KA, et al. Human umbilical cord mesenchymal stromal cells as an adjunct therapy with therapeutic hypothermia in a piglet model of perinatal asphyxia. Cytotherapy. 2021;23(6):521–535. https://doi.org/10.1016/j.jcyt.2020.10.005
- 39. Hsieh JY, Wang HW, Chang SJ, et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8(8):e72604. https://doi.org/10.1371/journal.pone.0072604
- 40. Guo ZY, Sun X, Xu XL, et al. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res. 2015;10(4):651–658. https://doi.org/10.4103/1673-5374.155442
- 41. Min H, Xu L, Parrott R, et al. Mesenchymal stromal cells reprogram monocytes and macrophages with processing bodies. Stem Cells. 2021;39(1):115–128. https://doi.org/10.1002/stem.3292
- 42. Xu J, Feng Z, Wang X, et al. hUC-MSCs exert a neuroprotective effect via anti-apoptotic mechanisms in a neonatal HIE rat model. Cell Transplant. 2019;28(12):1552–1559. https://doi.org/10.1177/0963689719874769
- 43. Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164(5):973–979.e1. https://doi.org/10.1016/j.jpeds.2013.11.036
- 44. Anderson PJ, Burnett A. Assessing developmental delay in early childhood—concerns with the Bayley-III scales. Clin Neuropsychol. 2017;31(2):371–381. https://doi.org/10.1080/13854046.2016.1216518